Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to assess the incidence of late rebleeding of ruptured intracranial aneurysms treated with detachable coils.
PATIENTS AND METHODS: A clinical follow-up study was conducted in 393 consecutive patients with a ruptured aneurysm treated with detachable coils between January 1995 and January 2003. Late rebleeding was defined as recurrent hemorrhage from a coiled aneurysm >1 month after coiling. One patient was lost to follow-up. Total clinical follow-up of the 392 patients who were coiled for ruptured cerebral aneurysms was 18,708 months (1559 patient years; median, 48 months; mean, 47.7 months; range, 0–120 months).
RESULTS: Four patients suffered late rebleeding from the coiled aneurysm at 8, 12, 30, and 40 months after coiling, respectively. Two of these patients died. Another patient died of probable rebleeding 4 months after coiling. The incidence of late rebleeding was 1.27% (5/393) and mortality of late rebleeding was 0.76% (3/393). The annual late rebleeding rate was 0.32%, and the annual mortality rate from late rebleeding was 0.19%. During the follow-up period, 53 coiled aneurysms in 53 patients (13%) were additionally treated: 35 aneurysms (8.9%) were additionally treated with coils, 16 aneurysms (4.1%) were additionally clipped, and 2 aneurysms (0.5%) were additionally treated with parent vessel balloon occlusion.
CONCLUSION: The late rebleeding rate after coiling of ruptured cerebral aneurysms is very low. Follow-up of patients with a coiled aneurysm is mandatory to identify aneurysms that need additional treatment after reopening.
In the past decade, coiling of ruptured intracranial aneurysms has proved to be a safe and effective treatment. Main concerns of this treatment are the substantial proportion of aneurysms that are initially incompletely occluded and the possibility of reopening of the coiled aneurysm over time with inherent risks of rebleeding (1, 2). It is assumed that angiographic follow-up and additional treatment when necessary is sensible to reduce the risks of recurrent hemorrhage (3).
The purpose of our study was to assess the incidence of late rebleeding in 393 patients with a coiled ruptured intracranial aneurysm during a median follow-up period of 51.5 months, following this strategy of rigorous angiographic follow-up and retreatment when necessary.
Patients and Methods
Patients
Between January 1995 and January 2003, 393 consecutive patients with aneurysmal subarachnoid hemorrhage (SAH) were treated with detachable coils. The indication for coiling of the ruptured aneurysm was assessed in a weekly joint meeting of 2 neurosurgeons, 2 neurologists, and 2 interventional neuroradiologists. During the study period, indication for coiling gradually changed from “high surgical risk” aneurysms to all aneurysms amenable for coiling, and as a consequence the proportion of aneurysms treated endovascularly rose from 21% in 1995 to 80% in 2002.
Characteristics of 393 patients with a ruptured aneurysm treated with coils were as follows: women, 275/395 (70%); men, 188/393 (30%); age, median of 52 years (mean, 52.9 years; range, 25–81 years); Hunt and Hess (HH) I–II, 249/393 (63%); HH III, 81/393 (21%); HH IV–V, 63/393 (16%); median time of treatment after SAH, 5 days (mean, 9.5 days; range, 0–80 days); median aneurysm size, 8 mm (mean, 9.2 mm; range, 2–35 mm). The locations of the 393 ruptured aneurysms treated with coils were anterior communicating artery, 123; basilar tip, 85; posterior communicating artery, 68; middle cerebral artery, 28; pericallosal artery, 15; carotid tip, 11; basilar trunk, 8; carotid ophthalmic segment, 8; posterior cerebral artery, 6; anterior choreoideal artery, 5; vertebral artery, 3; cavernous sinus, one; and carotid hypophyseal segment, one. Our institutional review board did not require its approval or patient informed consent for this retrospective study.
Coiling Procedure
Coiling of aneurysms was performed with the patient under general anesthesia. Before coiling or after placement of the first coil, a bolus of 2500 or 5000 IU of heparin was administered intravenously, followed by drip infusion of 1000 U of heparin per 500 mL infusion fluid during the intervention. Heparin was continued intravenously or subcutaneously for 48 hours after the procedure, followed by 80 mg of aspirin orally, daily for 3 months. Coiling was performed with Guglielmi detachable coils (GDC-10 and/or-18; Boston Scientific, Fremont, CA), and some large aneurysms were coiled with mechanically detachable coils (Detach 18; Cook Inc., Copenhagen, Denmark). The aim of the coiling was to obtain an attenuated packing of the aneurysm until no additional coil could be placed. Initial angiographic results of coiling were classified as complete occlusion (98%–100%), near-complete occlusion (90%–98%), or incomplete occlusion (<90%). Aneurysm occlusion was determined in consensus during the weekly joint meeting.
Follow-Up Schedule
Patients who survived the hospital admission period were scheduled for clinical follow-up in the outpatient clinic at 6 weeks and for angiographic follow-up at 6 and 18 months. Results of angiographic follow-up were classified in the same way as the initial postembolization occlusion. Incomplete occlusion at any point in time was considered an indication for further therapy, unless clinical or anatomic factors dictated otherwise. Clinical follow-up was assessed according to the Glasgow Outcome Scale (GOS) at every outpatient clinic visit and at every admission for follow-up angiography. Results and consequences of clinical and angiographic follow-up were discussed in the weekly joint meeting. Notes of the meeting were made by a secretary and implemented in the patient record. When appropriate, during the meeting a decision was made for the need for additional treatment or extended angiographic follow-up. From 2002 onward, patients with aneurysms with complete occlusion at 6-month follow-up angiography were generally discarded from extended angiographic follow-up. When additional treatment was performed, the result was evaluated in the weekly meeting.
Clinical Outcome Assessment
For the 393 consecutive patients with coiling of the ruptured aneurysm, all relevant clinical and imaging data were reviewed (M.S., W.J.v.R., G.B., and P.N., in consensus). In January 2005, 180 patients with follow-up angiograms or outpatient clinic visits of >3 months earlier were approached by telephone by one of us (M.S. or W.J.v.R.) with a standard questionnaire regarding the occurrence of rebleeding (severe headache that necessitated family doctor’s attention or hospital admission). For 24 elderly patients or patients with a known poor outcome at the last visit, the general practitioner, referring physician, or physician from the nursing home was contacted first. For all patients who died since the last visit, the family doctor was contacted to disclose the cause of death from the death certificate. For patients with low GOS scores 1–3 (death, persistent vegetative state, or severe disability), an effort was made to disclose the cause of the disabling neurologic deficit or death, in particular whether this was procedure related, disease related, or related to other factors.
Angiographic Outcome and Additional Treatments
A flow chart of initial and 6-month angiographic results and additional treatments in 393 patients with a ruptured aneurysm treated with coils is displayed in Fig 1. Initial postembolization occlusion of 393 aneurysms was (near) complete in 366 and incomplete in 27 aneurysms. In 9 operable incompletely occluded aneurysms in good grade patients clipping was performed within 1 month after coiling. Of 393 patients, 309 had a follow-up angiogram at median 6 months. Angiographic occlusion of these 309 aneurysms at 6 months was (near) complete in 248 and incomplete in 61 aneurysms. Of these 309 patients, 168 had extended angiographic follow-up for a total of 521 follow-up angiograms (132 patients had 2 follow-up angiograms, 28 patients had 3, and 8 patients had 4). Total angiographic follow-up was 5.438 months (453 years; median, 15 months; mean, 17.6 months; range, 2–84 months).
Flow chart of initial and 6-month angiographic results and additional treatments in 393 patients with a ruptured aneurysm treated with coils.
Of the 393 patients, 53 (13%) received additional therapy for the coiled aneurysm. Sixteen aneurysms (4.1%) were clipped after coiling, 9 before 6-month follow-up angiography, 7 after 6-month follow-up angiography, and 2 after second coiling. Thirty-five aneurysms in 35 patients (8.9%) were coiled more than once: 2 times (28), 3 times (4), 4 times (one), and 5 times (2). Parent vessel occlusion after coiling was performed in 2 patients (0.5%).
Statistical Analysis
For patients with late rebleeding after coiling the following patient and aneurysm characteristics were compared with patients without late rebleeding: patient age, gender, aneurysm size, aneurysm location, incomplete initial aneurysm occlusion, and incomplete aneurysm occlusion at 6-month follow-up angiography. Aneurysm locations were categorized in 4 groups: anterior cerebral artery, middle cerebral artery, carotid artery, and posterior circulation.
Fisher exact test was used for categorical data, and unpaired Wilcoxon test for continuous variables. P values <.05 were considered significant.
Results
Clinical follow-up was available for 392 of 393 patients (99.7%). One patient emigrated to another country and was lost to follow-up. Total clinical follow-up of the 392 patients who were coiled for a ruptured cerebral aneurysm was 18,708 months (1559 patient years; median, 48 months; mean, 47.7 months; range, 0–120 months).
Deceased Patients
During the total clinical follow-up period of median 48 months, 70 patients (17.8%) died. Of these 70 patients, 23 died of sequelae of SAH (15, HH IV–V; 4, HH III; and 4, HH I–II), 21 died of documented unrelated causes (malignancy, 7; cardiac disease, 5; infectious disease, 5; dementia, 2; clipping of an additional aneurysm, one; and procedural rupture during coiling of an additional aneurysm, one), 11 died of procedural complications of coiling, 6 died of early rebleeding of the coiled aneurysm (4), 4 died of complications of additional surgery of the coiled aneurysm, 2 died of CT-confirmed late rebleeding from the coiled aneurysm, 8–40 months after coiling, one died of brain stem compression of the coiled basilar tip aneurysm 4 years after coiling, and 2 died of unknown causes.
Both patients who died of unknown causes were found dead in bed. One patient was found to have died 4 months after coiling and was categorized as a likely late rebleeding because initial aneurysm occlusion had been incomplete. On further analysis, this patient was considered to have suffered late rebleeding. The other patient was found lifeless 36 months after coiling and was categorized as an unlikely rebleeding, because 6- and 18-month follow-up angiograms showed stable complete occlusion of the coiled aneurysm. On further analysis, this patient was considered not to have suffered a late rebleeding and the cause of death was categorized as unknown.
Late Rebleedings
Three patients died from a late rebleeding after coiling of a ruptured aneurysm (see “Deceased Patients” section). Two additional patients survived CT-confirmed late rebleeding from coiled aneurysms, 12 and 30 months after coiling. The incidence of late rebleeding was 1.27% (5/393) and mortality of late rebleeding was 0.76% (3/393). Annual late rebleeding rate was 0.32%, (5/1559 patient years; 95% confidence interval [CI] 0.12%–0.78%). Annual mortality rate from late rebleeding was 0.19% (3/1559 patient years; 95% CI 0.04%–0.60%).
Cases with Late Rebleeding
Case 1.
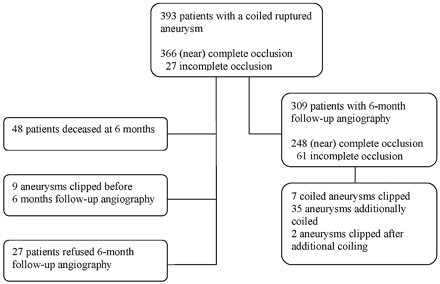
A 52-year-old man (HH grade I) was admitted after SAH from a 28-mm basilar tip aneurysm in 1995 (Fig 2). After insertion of 11 coils, repeatedly failed electrolytic detachment precluded insertion of additional coils and the aneurysm was left incompletely occluded. In the weeks that followed, the patient developed symptoms of brain stem compression, and occlusion of the basilar artery was considered. Test occlusions of the upper, mid, and lower basilar artery (J. Moret, Paris) were performed but not tolerated. A bypass from the external carotid artery to the P2 was constructed (C. A. Tulleken, Utrecht, the Netherlands), and both vertebral arteries were occluded with balloons. This resulted in subtotal occlusion of the aneurysm. Six months later, the patient suddenly died, and autopsy disclosed rebleeding from the basilar tip aneurysm.
Case 1, a 52-year-old man with a giant basilar tip aneurysm.
A and B, Giant basilar tip aneurysm partially occluded with coils. Detachment of additional coils failed.
B and C, Situation after bypass from the external carotid artery to the right P2 and bilateral vertebral artery occlusion. Only a small part of the neck is opacified with contrast material. Six months later, the patient died of autopsy-confirmed rebleeding.
Case 2.
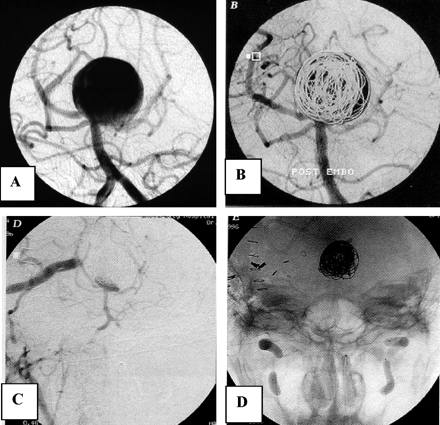
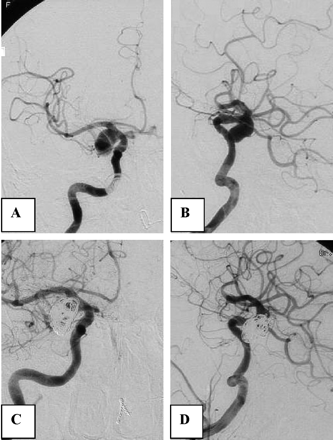
A 73-year-old woman was referred to our institution for coiling of a ruptured 10-mm basilar tip aneurysm (Fig 3). The aneurysm was coiled and almost completely occluded. The patient refused 6-month follow-up angiography. Twelve months after coiling, she was readmitted to another hospital after rebleeding from the coiled aneurysm and was referred for additional coiling. Angiography after the rebleeding showed that the aneurysm had enlarged, probably by resolution of intraluminal thrombus and the coil mesh was displaced and compacted. Additional coiling resulted in near complete occlusion of the aneurysm. The patient became dependent.
Case 2, a 72-year-old woman with a 10-mm ruptured basilar tip aneurysm and rebleeding 12 months after coiling.
A and B, Basilar tip aneurysm before (A) and after (B) coiling. Six-month follow-up angiography was refused.
C, Angiogram after rebleeding 12 months later. The aneurysm has enlarged and reopened.
D, Near-complete occlusion after additional coiling.
Case 3.
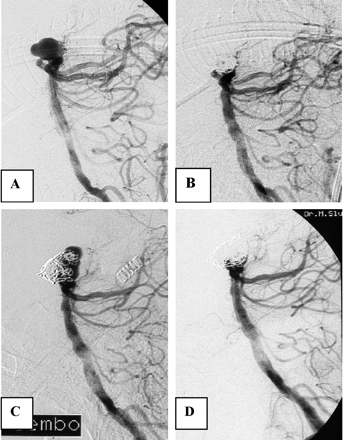
A 52-year-old woman (HH grade III) was admitted after rupture of a 16-mm posterior communicating artery aneurysm causing a predominantly intraventricular hemorrhage (Fig 4). The aneurysm was completely occluded with coils. Seven months later, the aneurysm showed partial reopening at the base and was coiled for a second time. Follow-up after second coiling was initially refused, but eventually, >2 years later, she agreed to undergo a follow-up angiogram that showed enormous enlargement and reopening of the aneurysm. At that time, a third coiling was refused. Three months later (40 months after initial coiling), she was readmitted with rebleeding, from which she died 1 day later.
Case 3, a 52-year-old woman with an intraventricular hemorrhage.
A and B, Angiograms before (A) and after (B) embolization show complete occlusion of large posterior communicating artery aneurysm.
C, Follow-up angiogram 7 months later reveals partial reopening of the base of the aneurysm.
D, After additional coiling complete occlusion, 6-month follow-up angiography after second coiling was refused.
E and F, Thirty months after second coiling, the patient suffered rebleeding, and angiography showed enlargement and reopening of the aneurysm.
Case 4.
A 76-year-old woman (HH grade I) was admitted after a SAH from a 17-mm posterior communicating artery aneurysm (Fig 5). The aneurysm was incompletely (about 80%) occluded with coils. She made an uneventful recovery, but 4 months later she was found dead in bed. Her family refused autopsy. This patient was considered to have suffered rebleeding from the coiled aneurysm.
Case 4, a 76-year-old woman with SAH from a large posterior communicating artery aneurysm.
A and B, Frontal (A) and lateral (B) views of carotid angiogram show large posterior communicating artery aneurysm.
C and D, After coiling residual filling of the aneurysm. Four months later, she was found deceased, possibly as a result of rebleeding.
Case 5.
A 64-year-old man (HH grade III) was admitted after SAH from an 18-mm anterior communicating artery aneurysm (Fig 6). The aneurysm was 90% occluded with coils. The neck was left open, because both A2s originated from the base of the aneurysm. Two additional small aneurysms on the left posterior communicating artery and left middle cerebral artery were not treated. Follow-up at 7 months showed partial reopening at the base of the aneurysm. Because the patient had a history of recent cardiac infarction and a recently diagnosed carcinoma of the tongue base, we advised against treating the large-neck remnant of the reopened aneurysm. Two years later, he was readmitted with rebleeding from the coiled aneurysm, and at that time additional coiling was performed with good result. The additional posterior communicating artery aneurysm was coiled in the same session. He made an uneventful recovery.
Case 5, a 64-year-old man with a ruptured anterior communicating artery aneurysm.
A, Angiogram reveals large anterior communicating artery aneurysm and additional small aneurysms on left posterior communicating artery and middle cerebral artery.
B, Near-complete occlusion after coiling. The additional aneurysms were not treated.
C, Follow-up angiogram 7 months later demonstrates enlargement of neck remnant. Additional coiling was not performed.
D, Angiogram after rebleeding shows further enlargement of neck remnant.
E, Angiogram after second coiling and coiling of the posterior communicating artery aneurysm.
Statistical Results
Median aneurysm size of patients with a late rebleeding (17 mm) was significantly larger than median aneurysm size of patients without a late rebleeding (8 mm; P = .0017). Three of 366 patients (0.82%) with an initial complete aneurysm occlusion had a late rebleeding versus 2 of 27 patients (7.41%) with an initial incomplete aneurysm occlusion. This difference was significant (P = .04). Patients with incomplete aneurysm occlusion at 6-month follow-up angiography suffered significantly more late rebleeding than did patients with complete aneurysm occlusion at 6 months (3/61 vs 0/248; P = .0074).
Median age of the 5 patients with a late rebleeding (63 years) did not differ significantly from those without late rebleeding (52 years; P = .0684). There was no significant difference in late rebleeding between genders (P = .64). There was no specific aneurysm location associated with late rebleeding.
Discussion
In this long-term follow-up study we found that rebleeding after coiling of ruptured cerebral aneurysms was infrequent with an incidence of 1.3% (5/392). The annual rebleeding rate was 0.35% and the annual mortality rate from late rebleeding was 0.19%. Risk factors for the occurrence of late rebleeding were large aneurysm size, initial incomplete aneurysm occlusion, and incomplete aneurysm occlusion at 6-month follow-up angiography. This low incidence of late rebleeding was obtained with a management strategy of meticulous angiographic follow-up and additional treatment of the coiled aneurysm in 13% of patients.
It is of note that late rebleeding in our series had less impact on patient outcome than early rebleeding: 6 patients died from early rebleeding after coiling (4), and 3 patients died from late rebleeding. Moreover, risk factors for early and late rebleeding are completely different. Risk factors for early rebleeding are small aneurysm size and an adjacent hematoma on initial CT scan, whereas risk factors for late rebleeding are large aneurysm size and incomplete aneurysm occlusion after initial embolization or at follow-up.
The low annual late rebleeding rate is in concordance with other studies that comprise fewer patient follow-up years. Byrne et al (5) reported an incidence of late rebleeding of 1.3% (4/317), and Raymond et al (6) reported an incidence of 1.1% (3/271).
Four of 5 rebleedings in our study probably could have been prevented. In one patient (case 1), failure of electrolytic detachment of coils precluded adequate aneurysm occlusion in 1995. This problem of early GDC design has been overcome in later years. One patient (case 2) refused 6-month follow-up angiography and angiography after the rebleeding showed a 50% reopening and enlargement of the aneurysm. It is likely that this reopening was present at 6 months, and additional coiling should have been performed then. One patient (case 3) with incomplete aneurysm occlusion 20 months after second coiling refused a third coiling. In retrospect, refraining from additional coiling in another patient (case 5) was a wrong decision.
The results indicate that angiographic follow-up is mandatory to timely detect reopening of coiled aneurysms and that additional therapy, when possible, should be performed. Additional coiling is then the therapy of choice because procedural complications are very low (3).
There is some controversy whether patients with a complete aneurysm occlusion at 6 months should have extended angiographic follow-up to detect late reopening of the aneurysm. In a previous study, we did not observe late reopening in aneurysms that were completely occluded at 6 months (7). Raymond et al (6), however, found that a considerable proportion of aneurysms reopen after 6 months. Because, in the present study, none of 248 aneurysms that were completely occluded at 6 months rebled during a very long follow-up period, it seems safe to refrain from extended angiographic follow-up in these cases. This discussion, however, has little relevance if MR angiography will replace conventional angiography for follow-up (8–10).
Data on recurrent aneurysm formation and late rebleeding after surgical clipping of ruptured aneurysms are scarce. Tsutsumi et al (11) reported 2.9% aneurysm regrowths after clipping on long-term follow-up angiography and David et al (12) found a late rebleeding risk of 1.9% per year after a mean follow-up period of 4.4 years; however, direct comparison of surgical and endovascular results is not valid because of differences in patient and aneurysm selection, proportion of patients with follow-up, and duration of follow-up.
With a strategy of coiling, angiographic follow-up, and additional treatment when necessary, great concern for late rebleeding after coiling of ruptured aneurysms is unfounded. The infrequent occurrence of late rebleeding also implies that, in the modification of coils with the aim to improve long-term stability of coiled aneurysms (13–16), the only step forward might be a reduction in reopening and retreatment rates. Influence on late rebleeding is likely to be low because late rebleeding rates can hardly become lower as they are. Moreover, these new coils must first prove to be as safe and effective as standard platinum coils before considering a widespread application (16).
Conclusion
The late rebleeding rate after coiling of ruptured cerebral aneurysms is very low. Follow-up of patients with a coiled aneurysm is mandatory to identify aneurysms that need additional treatment after reopening.
References
- Received February 21, 2005.
- Accepted after revision May 18, 2005.
- Copyright © American Society of Neuroradiology