Abstract
SUMMARY: Herceptin (trastuzumab) is a human monoclonal antibody that interferes with the HER2 receptor. It is currently the only FDA-approved therapeutic antibody for HER2-positive breast cancer. This article will present the mechanism at action as well as the clinical role at this monoclonal antibody.
Abbreviations
- FDA
- US Food and Drug Administration
- HER, HER2, HER3, and HER4
- human epidermal growth factor receptors
Trastuzumab (Herceptin) is humanized monoclonal antibody that interferes with the HER2 receptor. It is currently the only FDA-approved therapeutic antibody for HER2-positive breast cancer. The targeting of HER overexpression was considered a major innovation to the concept of “personalized medicine.”
Mechanism of Action
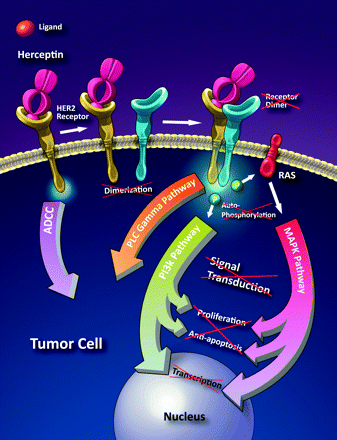
HER are cell membrane-bound glycoproteins, comprising 4 distinct receptors: HER1, HER2, HER3, and HER4.1–3 These receptors are divided into 3 regions: an extracellular ligand-binding region, an intracellular region with tyrosine kinase activity, and a region that spans the cell membrane and anchors the receptor to the cell. Ligand binding to the extracellular domain promotes formation of dimmers, homodimers (between monomers of same receptor), or heterodimers (between the bound receptor and other members of the HER family), activating tyrosine kinase, and triggering a cascade of complex cell biochemistry that regulates various cell functions such as cell proliferation, angiogenesis, apoptosis, adhesion, and motility (Fig 1 ).2,3
Schematic illustration showing the proposed mechanism of trastuzumab. Herceptin is a monoclonal antibody that binds to the extracellular portion of the HER2 gene, preventing dimerization and the cascade that leads to the expression of growth factors. It also induces apoptosis through antibody-dependent cellular cytotoxicity. Illustration by Carolyn Nowak.
The HER2 gene (HER2/neu, ErbB2 gene) is located on the long arm of chromosome 17 and is amplified (overexpressed) in 20%–30% of early-stage breast cancers. The triggering mechanism of most HER receptors is binding of a mitogen to the extracellular ligand portion of the HER receptor. However, there is no known mitogen (ligand) for HER2. Overexpressed HER2 sends signals without mitogen arriving and binding to any receptor. These signals promote invasion, survival, and angiogenesis of tumoral cells.4
Trastuzumab binds to domain IV of the extracellular segment of the HER2.4 This process prevents dimerization, causing cell arrest during the G1 phase. Some of the therapeutic effect may also be due to downregulation of HER2. These mechanisms cause disruption of receptor dimerization, which reduces signaling pathways and results in cell-cycle arrest.
Indication and Usage
Trastuzumab has had a substantial impact in the treatment of HER2 overexpressing (HER2-positive) node-positive breast cancer.1,3 Adjuvant therapy with trastuzumab and chemotherapy (paclitaxel, doxorubicin, and cyclophosphamide) has been shown to increase both survival and response rates, in comparison with trastuzumab alone.1,4 Trastuzumab is also indicated with paclitaxel for first-line therapy for patients with HER2-positive metastatic breast cancer. Trastuzumab as a single agent is also indicated for second- and third-line therapy for patients who are HER2-positive and have previously received 1 or more chemotherapy agents.
Administration and Side Effects
The most common adverse reactions are fever, nausea, vomiting, diarrhea, infections, cough, headache, fatigue, dyspnea, rash, neutropenia, anemia, and myalgia.5 One of the significant complications of trastuzumab is its effect on the heart, and it is associated with cardiac dysfunction in 2%–7% of cases.5 Specifically, patients are at risk for left ventricular dysfunction and congestive heart failure. Regular cardiac screening with either a multigated acquisition scan or echocardiography is commonly undertaken during the trastuzumab treatment period. Serious infusion reactions (angioedema, anaphylaxis) and pulmonary toxicity (pneumonitis, acute respiratory distress syndrome) have also been reported within 24 hours of administration.
Economic and Clinical Issues
The average wholesale price for trastuzumab is $2930 for 440 mg.6 For 70 kg, each 2 mg/kg dose would cost approximately $1000, and a yearly regimen would cost $50 000. About 5–10 women per 100 000 population have metastatic breast cancer, between 90 and 100 per 100 000 have localized breast cancer, and around 40 per 100 000 have regional breast cancer.7 Thus, with a US female population of >143 000 000, approximately 7000–14 000 women have metastatic breast cancer, 130 000–140 000 have localized breast cancer, and 60 000 have regional breast cancer.8 If trastuzumab is to be a standard part of the adjuvant regimen for HER2-positive patients, the cost of treating this population could increase by almost $1 billion for trastuzumab alone. There would also be additional cost for treating heart failure associated with administration of the medication.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- Received February 7, 2011.
- Accepted after revision February 8, 2011.
- © 2011 by American Journal of Neuroradiology