Abstract
BACKGROUND AND PURPOSE: Patients with LS have an inborn growth hormone resistance, resulting in failure to generate IGF-1. The purpose of this study was to evaluate the size of the eye and orbit in LS.
MATERIALS AND METHODS: We retrospectively reviewed the MR imaging of the brain in 9 patients with LS for the following parameters: axial diameter of the globe, interzygomatic distance, perpendicular distance from the interzygomatic line to margins of the globe, medial-to-lateral diameter of the orbit at the anterior orbital rim, distance from the anterior orbital rim to the anterior globe, maximal distance between the medial walls of the orbits, lateral orbital wall angle, lateral orbital wall length, and mediolateral thickness of the intraorbital fat in the most cranial image of the orbit. All measurements were made bilaterally. Twenty patients referred for MR imaging for unrelated reasons served as control subjects.
RESULTS: Compared with the control group, the patients with LS had a significantly smaller maximal globe diameter and shallower but wider orbits due to a shorter lateral wall, a smaller medial distance between the orbits, and a larger angle of the orbit. The ratio between the most anterior orbital diameter and the globe was greater than that in controls. The position of the globe was more anterior in relation to the interzygomatic line.
CONCLUSIONS: Shallow and wide orbits and small globes relative to orbital size are seen in LS and may be secondary to IGF-1 deficiency.
Abbreviations
- GH
- growth hormone
- IGF-1
- insulin-like growth factor 1
- LS
- Laron syndrome
- NS
- not significant
LS (Online Mendelian Inheritance in Man, catalogue no. 262500) also known as congenital GH insensitivity or resistance, is an autosomal recessive disease caused by deletions or mutations in the GH receptor gene or the postreceptor pathways.1,2 Because of the defects in GH signal-intensity transmission, individuals do not generate IGF-1, the anabolic effector hormone of pituitary GH and, consequently, fail to respond to GH of either endogenous or exogenous origin. The clinical features of LS are indistinguishable from those of untreated isolated GH deficiency; however, patients have high serum levels of GH and very-low-to-undetectable serum levels of IGF-1.1,2
Clinically, LS is manifested by progressive dwarfism, with final stature ranging from 108 to 136 cm in women and 116 to 142 cm in men.1,2 Acromicria and organomicria are other common features. The circumference of the head is small, and the facial structures are underdeveloped. There may be an absence or underdevelopment of the frontal and maxillary sinuses and small sphenoid sinuses and mastoid antra.3 Although both skeletal and sexual maturation are retarded, affected individuals eventually mature and have full reproductive ability.1,2 As adults, they become more and more obese, with hypercholesterolemia and insulin resistance.1 With the development of biosynthetic IGF-1, treatment is now feasible, but only a portion of children receive it owing to its high cost.
Patients with LS serve as a unique model for the investigation of the effects of GH/IGF-1 on the eye and orbit. A recent ophthalmologic study reported that in patients with LS, the axial lengths of the eye and anterior chamber are shorter than those in healthy controls.4 To the best of our knowledge, however, imaging evaluations of these structures have not been performed in patients with LS. The purpose of the present study was to assess, by MR imaging, the size of the eye and the orbit and the relationship between these in LS.
Materials and Methods
A comparative retrospective case series design was used. The study was conducted in a university-affiliated tertiary medical center, and the protocol was approved by the institutional review board, with a waiver of informed consent. The files of the Imaging Department were searched for all patients with LS who underwent MR imaging of the brain from 1999 to 2007. The diagnosis of LS was based on findings of severe short stature, high basal levels of GH with low serum levels of IGF-1, lack of response to exogenous GH administration, and molecular studies of the GH receptor genes.1 Only patients who had not received replacement therapy with IGF-1 were included. In all cases, the MR imaging examinations had been performed because of clinical symptoms unrelated to the eye or orbit, such as headache and dizziness, and for evaluation of mental retardation.
Owing to the retrospective nature of the study, the MR imaging equipment included both 0.5T and 1.5T systems, depending on the time of examination. The measurements for the present study were made on the axial images, either T1- or T2-weighted, according to the preference of the reviewing radiologist.
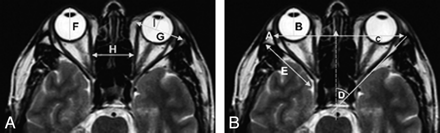
We measured the following parameters (Fig 1): axial diameter of the globe (F), interzygomatic distance (A), perpendicular distance from the interzygomatic line to the anterior margin of globe (B), perpendicular distance from the interzygomatic line to the posterior margin of the globe (C), medial-to-lateral diameter of the orbit at the anterior orbital rim (G), distance from the anterior orbital rim to the anterior globe (I), maximal distance between the medial walls of the orbits (H), lateral orbital wall angle (D), lateral orbital wall length (E), and mediolateral thickness of the intraorbital fat in the most cranial image of the orbit. We calculated the ratio of the length of the lateral orbital wall to the diameter of the globe and the ratio of the diameter of the orbit at the anterior orbital rim to the diameter of the globe. All measurements were made bilaterally.
Axial T2-weighted images of the orbits. The measured parameters are demonstrated as follows: A, interzygomatic distance; B, distance from line A to the anterior globe; C, distance from line A to the posterior globe surface; D, angle of the orbit; E, length of the lateral wall of the orbit; F, diameter of the globe; G, anterior diameter of the orbit; H, interorbital distance; and I, distance from line G to the anterior surface of globe.
The findings were compared with a control group of patients retrieved from the radiology files who underwent MR imaging of the brain for reasons other than investigation of the eyes or orbits. Those with abnormal findings in this region were excluded.
Statistical Analysis
Statistical analysis was performed by using BMDP statistical software (Statistical Solutions, Saugus, Massachusetts). The calculated means and SDs for the left and right eyes were combined because the various measurements were very similar bilaterally. Because the sample was relatively small, we compared the 2 groups by using the Mann-Whitney nonparametric test. A P value of ≤.05 was considered statistically significant.
Results
The study group consisted of 9 patients (5 men, 4 women, 36–68 years of age; mean, 45.5 ± 9.2 years), and the control group consisted of 20 patients (4 men, 16 women; 21–58 years of age; mean, 38.4 ± 13 years). There was no significant difference in age between the groups.
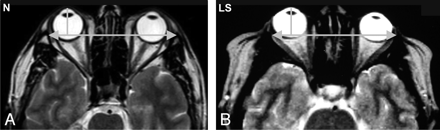
The orbital measurements are shown in the Table. Compared with the control subjects, the patients with LS had a significantly smaller maximal globe diameter, shallower but wider orbits due to a shorter lateral wall, a smaller medial distance between the orbits, and a larger angle of the orbit. There was no significant difference in the most anterior diameter of the orbit. Thus, the ratio between the most anterior orbital diameter and the globe was greater in the study group than in the controls. The ratio between the length of the lateral wall of the orbit and the globe diameter was not significantly different in the LS group, and the position of the globe was more anterior in relation to the interzygomatic line but not in relation to the anterior orbital rim (Fig 2).
Axial T2-weighted images of the orbits. Note that in LS, the orbits are shallower and the globe is more anterior in relation to the interzygomatic line. N indicates healthy control subject.
Orbital measurements in patients with LS compared with healthy controls
Discussion
Patients with LS have a characteristic physiognomy: prominent forehead, decreased vertical dimension of the face, hypoplastic nasal bridge, and small maxilla and mandible. Their appearance reflects the underdevelopment of the facial bones due to lack of IGF-1.5 The small orbits are part of the impaired facial growth. Earlier studies reported a smaller-than-normal distance between the temporomandibular joints by cephalometric measurements6 and underdeveloped paranasal sinuses.3
The present study focused on evaluation of the orbits in LS by MR imaging and adds new data on their shape and size. We found that the bony orbits in the study group were smaller and shallower but wider than those in the control subjects without LS (Table). The lateral wall length was smaller, and the angle of the orbit was larger. There was a lesser medial distance between the orbits than that in controls, with no significant difference in the most anterior orbital diameter (Fig 2).
The significantly smaller maximal diameter of the globe in the patients with LS is concordant with a recent ophthalmologic study, wherein the axial lengths of the eye and the anterior chamber were found to be smaller in patients with LS than in control subjects.4 Children with LS who were treated with IGF-1 had a larger globe than untreated children.4 Parentin and Perissutti7 found that in children with GH deficiency, the axial length of the globe is shorter than normal. Adult height is independently related to ocular dimensions, such that taller persons are more likely to have longer globes.4
Besides regulating the growth of the globe, the GH/IGF-1 axis plays multiple roles in the normal development of the eye. It is involved in scleral growth, optic nerve growth, and vascularization of the retina. Accordingly, optic nerve hypoplasia and pseudopapilledema, as well as reduced retinal vascularization, are all described as possible signs of GH deficiency.8 However, our patients did not have a congenital anomaly of the eye and, on detailed ophthalmologic examination, showed only a tendency to hyperopia due to the small diameter of the globe.
Postnatal growth of the orbit is dependent on normal growth of the globe.9 Both grow considerably during the first years of life. About two-thirds of the postnatal increase in ocular axial length takes place within the first 24–30 months of life; thereafter, growth decelerates.10 The time at which final eye size is attained is still controversial. The orbit reaches >85% of adult size by 5 years of age.11 In our study group, the ratio of the diameter of the orbit at the orbital rim to the globe diameter was greater than that in the control group. Although the position of the globe was normal relative to the anterior orbital rim, it was significantly more anterior than normal relative to the interzygomatic line. Compared with values in the literature,12 the position of the globe was compatible with exophthalmus (defined as a distance of >21 mm from the interzygomatic line to the anterior globe12) in only 3 patients.
The combined findings of the anterior position of the globe and a relatively large orbit suggest that both may be explained by an excessive amount of fat. Patients with GH insensitivity are indeed obese, and marked obesity may cause exophthalmus.13 However, we measured the intraorbital fat below the orbital roof and found it be less thick than that in the healthy controls. We have no good explanation for this finding. Perhaps volumetric measurements of the orbital fat, which were not feasible due to the retrospective nature of the study, would have yielded different results. Another reason for the relatively smaller globe may be the complexity of ocular growth regulation. The mechanism involves many local factors, not exclusively IGF-1, which could result in a differential influence of IGF-1 on the globe and orbit.4
The interorbital distance increases with age. Its small size in LS is probably a direct effect of deficient IGF-1 as well as part of the impaired growth of the upper face. The ocular axis (the angle between the optic nerves) diminishes slightly from childhood to adulthood.9 We did not measure this angle, but the orbital angle was found to be larger in the patients with LS.
The refraction of the eye depends on 3 variables and their interaction: corneal power, lens power, and axial length. The human eye is programmed to achieve emmetropia in youth, despite the changes in these variables. The eye grows rapidly during the first year of life, concomitant with flattening of the cornea and a decrease in the power of the lens. After 6 years of age, refraction is modified mainly by increases in axial length.8 In an earlier clinical study of 12 untreated patients with LS, refraction examination yielded only a tendency toward hyperopia related to the small globe axis, thick lens, and steep corneal curvature.4 These differences from normal values in the literature reflect the influence of IGF-1 on the various components of the eye. There is anecdotal evidence that the small anteroposterior dimension of the orbit and the anterior position of the globe may manifest clinically. Ophthalmologic examination revealed increased proptosis and midface hypoplasia, most probably associated with small orbits (Z. Laron, personal communication, 2005).
The major weakness of the study is its retrospective design. We could neither perform volumetric measurements of the bony orbit and its content, which are more reliable, nor base our measurements on high-resolution studies of the orbits.
In conclusion, shallow and wide orbits and small globes relative to orbital size are seen in LS and may be secondary to IGF-1 deficiency. Further studies are needed to elucidate the influence of IGF-1 on ocular growth.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- Received October 1, 2010.
- Accepted after revision December 22, 2010.
- © 2011 by American Journal of Neuroradiology