Abstract
BACKGROUND AND PURPOSE: A growing body of evidence suggests the involvement of the brain in FM. The purpose of this proton MRS study was to test the hypothesis that there are metabolic alterations in some brain regions processing pain (VLPFC and thalamus) in patients with FM compared with HC.
MATERIALS AND METHODS: Twelve patients with FM (30–54 years of age; mean age, 43.2 years), and 12 HC, matched for age and sex, underwent 1 session of single-voxel MRS performed on a 3T MR imaging scanner. MRS spectra were acquired with a PRESS for localization. The raw data from each spectrum was evaluated with an LCModel. T tests were used to evaluate differences of brain metabolites between groups. The Pearson correlation tested the relationship of metabolite ratios and clinical symptoms.
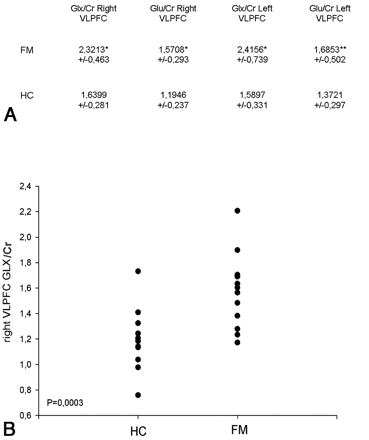
RESULTS: Glx/Cr and Glu/Cr ratios within the VLPFC of both sides were significantly higher in patients than in HC (P < .01). No significant differences of metabolites between groups were found in the thalami. Positive correlations were found between Glu/Cr in the left thalamus and the VAS for pain (r = 0.730, P = .007) and between mIns/Cr in the right VLPFC and the VAS for pain (r = 0.607, P = .037) and the FIQ (r = 0.719, P = .008).
CONCLUSIONS: The presence of elevated Glu/Cr levels in VLPFC strengthens the opinion that a complex neurophysiologic imbalance of different brain areas involved in pain processing underlies FM. These data may be useful in the diagnosis and development of more effective pharmacologic treatments.
Abbreviations
- CBF
- cerebral blood flow
- Cho
- choline-containing compounds
- Cr
- creatine and phosphocreatine
- FIQ
- Fibromyalgia Impact Questionnaire
- FM
- fibromyalgia
- fMRI
- functional MR imaging
- FSE
- fast spin-echo
- FSPGR
- fast-spoiled gradient recalled
- FWHM
- full width at half maximum
- Glu
- glutamate
- Glx
- glutamate plus glutamine
- HC
- healthy controls
- mIns
- myo-inositol
- MRS
- MR spectroscopy
- NAA
- N-acetylaspartate
- PET
- positron-emission tomography
- PRESS
- point-resolved spectroscopy sequence
- SPECT
- single-photon emission CT
- tNAA
- total-NAA (N-acetylaspartate + N-acetylaspartylglutamate)
- VAS
- Visual Analog Scale
- VLPFC
- ventrolateral prefrontal cortex
FM is a chronic pain disorder, with a prevalence between 2% and 4% in industrialized countries,1 characterized by widespread nonarticular pain and diffuse tenderness to light palpation. Patients may also exhibit chronic fatigue, disturbed sleep, and stiffness.2 Furthermore, there is a variety of neurologic phenomena associated with the disorder such as autonomic dysfunction3 and cognitive disturbances, including impaired concentration and deficits in short-term memory.4 The subjective nature and the extreme heterogeneity of FM symptoms have led to a long-standing debate regarding the nature of this condition,5,6 though the etiology is not yet known.
Human and comparative research of chronic pain has yielded new insights into the pathophysiology of FM pain, suggesting that it may be associated with a brain dysregulation of nociceptive and pain processes. Functional neuroimaging studies by using SPECT, fMRI, and PET have been largely for evaluation of patients with chronic pain and FM.7–16 In particular, PET and SPECT investigations have detected differences in CBF between patients with FM and HC in many areas (the insula, caudate nucleus, anterior cingulate cortex, and other prefrontal areas including the dorsolateral and medial prefrontal cortices) with major evidence at the thalamic level. In addition, fMRI studies investigating the cognitive control of pain have demonstrated that increased activity in the VLPFC was negatively correlated with the subjective intensity of pain, suggesting a critical role for this brain region in the downregulation of pain.17–20 Hence, potential dysfunctions of this region may represent an important pathophysiologic mechanism underlying FM, but to date, this issue has not been investigated.
Recently, some MRS studies21–27 have demonstrated alteration of cerebral metabolites in patients with FM, and though the results are not homogeneous, they strengthen the hypothesis of a neural dysfunction secondary to the pain processing.
The purposes of our study were to test the hypothesis that there are metabolic alterations in the thalamus and VLPFC of patients with FM compared with HC and that the results could correlate with clinical symptoms.
Materials and Methods
Subjects
A total of 12 patients (11 women, 1 man; 30–54 years of age; mean age, 43.2 years) and 12 HC matched for sex and age (28–56 years of age; mean age, 41.3 years) took part in the study. All patients were recruited from the Rheumatology Unit, S. Orsola-Malpighi Hospital of Bologna. Patients who met the American College of Rheumatology criteria for diagnosis of FM,2 without any comorbidity (other rheumatologic diseases and/or central nervous system pathologies) were recruited. Written informed consent was obtained from all patients.
All patients suspended pharmacologic therapies that are known to affect brain function a week before the study to avoid possible biases, with the exception of acetaminophen, which was permitted to control pain. None of the control subjects took drugs that affected cognitive functions or brain metabolites. Immediately before the examination, patients completed the FIQ28 (a self-report measure that evaluates the overall impact of FM over many dimensions such us function, pain level, fatigue, sleep disturbance, and psychological distress). Furthermore, the number of tender points (specific muscular regions that are painful when manually palpated with 4 kg of pressure) was assessed in all patients. To measure pain, we used a marked 100-mm horizontal VAS, ranging from 0 (no pain) to 100 (severe pain).
MR Imaging/Proton MRS
We performed all MR imaging and localized single-voxel MRS measurements by using a 3T whole-body scanner (Signa EXCITE; GE Healthcare, Milwaukee, Wisconsin) equipped with an 8-channel standard phased-array head coil. T2-weighted FSE sequences (TR, 4020 ms; TE, 106 ms; NEX, 1; 512 × 512 matrix; 4-mm sections; FOV, 256 mm) were acquired in the axial and coronal planes, while a 3D T1-weighed FSPGR sequence (TE, 3.7 ms; TR, 9.8 ms; TI, 750 ms; flip angle, 12°; NEX, 1; 256 × 256 matrix; 1.0-mm sections; FOV, 256 mm; bandwidth, 25.00 HZ/pixel) was obtained with reconstructions on axial and coronal planes.
MRS spectra were acquired with a PRESS for localization (TR, 2000 ms; TE, 35 ms; 128 acquisitions), and a 3-pulse chemical shift selective suppression sequence to provide water suppression. For each spectrum, we collected 16 additional acquisitions with unsuppressed water for phase correction of the metabolite spectra. We used automated optimization of gradient shimming, transmitter pulse power, and water suppression. In all cases, the quality of the shimming obtained in the voxel was controlled by the spectral line width (FWHM in hertz) of the unsuppressed water, obtained by the automated optimization sequence before scanning.
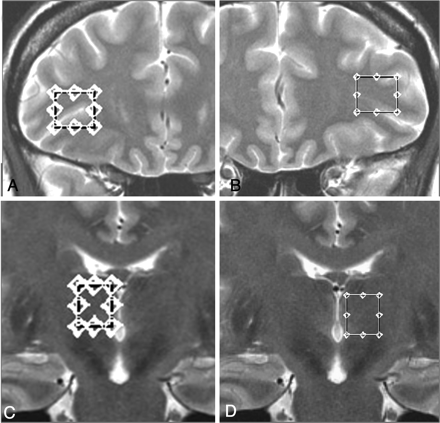
The volumes of interest were placed on the thalamus and VLPFC (1.8–2.1 cm3, respectively) from both sides on 3D FSPGR sagittal and T2-weighted FSE axial and coronal planes with attention to minimize sampling contaminations by bone, vascular, and CSF structures (Fig 1).
T2-weighted FSE images showing voxel placement in the left and right VLPFCs (A and B) and thalami (C and D) of a subject with FM.
Analysis of Spectra
The raw data from each single-voxel spectrum were evaluated with LCModel, Version 6.1 (Stephen Provencher, Oakville, Ontario, Canada29). This method exploits the full spectroscopic information of each metabolite and not just isolated resonances. Values for tNAA, Cho, Cr, mIns, Glu, and Glx were calculated as ratios to an internal standard, the Cr level, in the form of tNAA/Cr, Cho/Cr, mIns/Cr, Glu/Cr, and Glx/Cr.
Statistical Methods
We analyzed tNAA/Cr, Cho/Cr, mIns/Cr, Glu/Cr, and Glx/Cr group ratios of both the VLPFC and thalami with the Student t test (significance was inferred at P < .05). The Pearson correlation was used to determine significant relationships between metabolite ratios and the FIQ, the VAS for pain, and the number of tender points (P < .05 was accepted as statistically significant).
Results
Conventional morphologic MR imaging results were normal for all subjects included in the study.
Spectra of good quality were achieved in all cases. In 1 patient, it was impossible to acquire the spectra of the left VLPFC. The final FWHM values (reported as the mean ± SD) estimated by LCModel were 0.044–0.009 ppm for the frontal regions (right and left VLPFC) and 0.050–0.01 ppm for the thalami. Only Glx/Cr and Glu/Cr ratios of the left and right VLPFC were significantly different between patients and HC. In particular Glx/Cr and Glu/Cr ratios were higher in the patients than in controls (P < .01) (Figs 2 and 3). There were no significant differences between the left and right sides. All the other principal metabolite ratio results were within the normal range of the control group. No significant differences of metabolite ratios were found in both thalami (Table).
A, Right and left VLPFC Glx/Cr and Glu/Cr ratios of patients with FM and HC. The differences were P < .01 (asterisk) and P < .05 (double asterisks). B, Graph showing the distribution of Glx/Cr ratios in the right VLPFC in patients with FM and HC. Group differences: P = .0003.
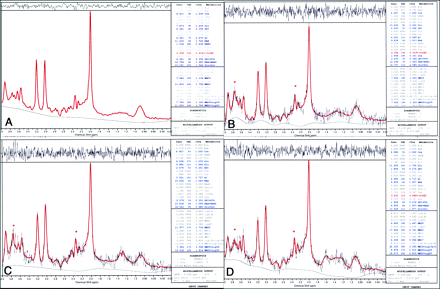
Proton MRS spectra from the right VLPFC fit with the LCModel in 1 control (A) and 3 patients (B−D). Red trace (asterisk) indicates resonance from 2 Glu γ proton resonances at 3.74 and 2.35 ppm.
Metabolite ratio values of the VLPFC and thalami in patients with FM and HCa
A significant positive correlation was found between Glu/Cr levels in the left thalamus and the VAS (r = 0.730, P = .007; Fig 4), and between mIns/Cr in the right VLPFC with the VAS (r = 0.607, P = .037) and FIQ (r = 0.719, P = .008; Fig 5). A significant positive correlation was also found between Glx/Cr and mIns/Cr in the right VLPFC (r= 0.585, P = .046). No correlations were found among metabolite ratios and the number of tender points.
Significant correlations between metabolite ratios and clinical symptoms. The Pearson moment correlation of Glu/Cr in the left thalamus and clinical pain scores on the VAS.
Significant correlations between metabolite ratios and clinical symptoms. The Pearson moment correlation of the mIns/Cr ratio in the right VLPFC and the FIQ.
Discussion
Fibromyalgia is characterized by a combination of symptoms including chronic widespread pain, chronic fatigue, and a variety of cognitive disturbances such as impaired concentration and deficits in short-term memory. Although converging data support the hypothesis that dysregulation in brain regions processing pain has a primary role in the pathophysiology of FM, the detailed mechanisms underlying this disorder are still unknown. Our results clearly demonstrate a dysfunction of the VLPFC in FM as evidenced by an increase of Glu/Cr and Glx/Cr ratios in the left and right VLPFCs of patients with FM compared with controls. Although we assessed the ratios of Cr, we suppose that the differences in the Glu/Cr and Glx/Cr ratios we found between patients and controls are driven by increased levels of Glu and Glx, respectively, and not by a reduction of the Cr, because the other metabolite ratios did not show differences between patients and controls (Table).
Several functional imaging studies have been published on FM but few used MRS to investigate metabolic alterations. Although the results of these studies are not homogeneous,21–27 because of the different MRS protocols, magnetic fields used, and areas of study, in 4 of these, alterations of the glutamatergic system were detected.
Glu is a largely diffused neurotransmitter in the brain and spinal cord, and it plays important roles in the normal function of the central nervous system. Dysregulation in the Glu neurotransmission has also been implicated in the genesis of several neurologic conditions. The accumulation of extracellular Glu is toxic to neurons. If removal of Glu from the synapse does not keep pace with accumulation, neuronal damage may occur.30 There are many studies on the implication of Glu in nociception.31–33 In particular, an increase of Glu at the level of anterior cingulate cortex was demonstrated by Mullins et al34 in association with painful stimuli, and these variations correlated with the perceived pain intensity. Our results confirm this glutamatergic alteration, and the increase of Glx/Cr and Glu/Cr in both VLPFCs and not in thalami adds new insight into the physiopathology. In fact, the involvement of the right VLPFC in cognitive down-modulation of pain35 and of the left VLPFC in cognitive control of memory has been consistently shown.36 In particular, an involvement of reappraisal mechanisms in mediating the placebo effect has been indicated by neuroimaging studies18–21 that have consistently shown activation in the VLPFC correlated with the reduction of pain during placebo.
Moreover, cortical morphologic and functional changes associated with neuropathic pain were detected in the medial prefrontal cortex in a rat model.37 Hence, we suppose that a neuronal pain-related dysfunction in the VLPFC could reduce the analgesic control that this area plays on pain perception, and we could interpret the increase of Glu/Cr and Glx/Cr as a secondary dysfunctional response of cognitive modulation that is produced by or interacts with pain processing in patients with FM. This could determine the reduced threshold to “the painful” stimuli (eg, pressure, noise) evident in FM syndrome. We also found positive correlations between mIns/Cr in the right VLPFC with the VAS (r = 0.607, P = .037) and FIQ (r = 0.719, P = .008) and a significant positive correlation between Glx/Cr and mIns/Cr at the same level. mIns is thought to be a glial marker and an organic osmolyte that plays a major role in the osmoregulation of astrocytes.38 We interpreted our findings as local changes reflecting the effect of Glu. This positive relation between mIns/Cr with the VAS and FIQ could be used to monitor therapy responses, and further studies are needed to confirm this relationship.
In our patients, the increase of Glx/Cr and Glu/Cr was also present in the left VLPFC. Although no relation between metabolite ratios of this side was found with the VAS, FIQ, or number of tender points, we think that this alteration could correlate with/or be responsible for other symptoms of patients with FM, such as deficits of attention and working memory,4 which are strictly related to activity on the left VLPFC. Additional studies could explore metabolic and physiologic changes in this brain region.
A further important structure with documented involvement in FM is the thalamus. It has been demonstrated in several PET and SPECT studies that detected differences in CBF between patients and controls in many areas including the thalamus.10–13 Although there were no differences between patients and controls at this level, we found a strong significant positive correlation between Glu/Cr levels in the left thalamus and the VAS (r = 0.730, P = .007). This result strengthens a recently published MRS study,26 in which patients with more pain (high VAS score) showed higher Glx levels in the left thalamus, though without significant correlation. Our result could reflect the role of the thalamic structures in the processing of pain signal intensity. However, both in our study and in the previous MRS studies26,27 that explored this region, no differences between patients and HC were found in metabolites and their ratios, even though alterations of CBF are well documented at this level. In our opinion, this information suggests that the thalamus is not primarily involved in this disorder but probably interacts with other cognitive areas that process pain such as the VLPFC. Further fMRI studies are needed to confirm these findings.
Limitations
Although significant, the results of this spectroscopy study could suggest the use of a more specific method to evaluate the Glx complex and to quantify Glu. We were only able to use single-voxel PRESS.
Even though LCModel also permits an accurate absolute metabolite quantification, we decided to use ratio quantification because in our experience, the ratios are more constant between groups.
Conclusions
MRS is a useful instrument for the study of cerebral metabolism in patients with FM. The metabolic alteration, consisting of an increase of Glu/Cr, could indicate a possible pathogenesis of the disease. Elevated Glu levels have been reported in the CSF of patients with FM,39 and this molecule could be responsible for or be related to the augmented pain transmission observed in FM.15
The absence of metabolic alterations at the thalamic level and the presence of elevated Glu/Cr levels in the VLPFC strengthen the opinion that in patients with FM, there is a complex neurophysiologic imbalance of multiple areas involved in pain processing, with consequent central sensitization.
This could also help to resolve the long-standing debate regarding the legitimacy of FM,40 which originated from the subjective nature of FM symptoms in the absence of an objective diagnostic approach. Our findings provide direct evidence of abnormal glutamatergic metabolism in FM, which may be useful in diagnosis and future development of more effective pharmacologic treatments. This information can be also useful for developing diagnostic tests and measuring therapeutic responses.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- Received October 21, 2010.
- Accepted after revision January 11, 2011.
- © 2011 by American Journal of Neuroradiology