Abstract
SUMMARY: The Outreach DAC is an intermediate-sized catheter designed for use with the Merci clot retriever in acute stroke. We investigated its utility as an adjunctive device during AVM pedicle embolization. In the authors' opinion, the DAC provided additional guide-catheter and microcatheter support, improved selective angiographic visualization of AVM angioarchitecture, aided microcatheter removal from its embedded position in the AVM Onyx cast, and enhanced local microcatheter control and safety, compared with embolization with the guide and microcatheter alone.
ABBREVIATIONS:
- AVM
- arteriovenous malformation
- DAC
- Distal Access Catheter
- DSA
- digital subtraction angiography
- n-BCA
- n-butyl 2-cyanoacrylate
Endovascular embolization is frequently used in the treatment of brain AVMs. These are occluded by injecting an adhesive embolizing agent, such as n-BCA, or a nonadhesive embolizing agent, such as Onyx (ev3/Covidien Vascular Therapies, Mansfield, Massachusetts). The complexity and location of many brain AVMs1⇓–3 cause the neurovascular interventionist to contend with large distances between the guide catheter and microcatheter, reducing the amount of support available for the microcatheter and guide for navigation. Particularly with the use of Onyx as an embolizing agent, encasement of the microcatheter in the Onyx cast can be seen. Furthermore, routine guide catheter runs obtained during DSA results in opacification of all cerebral vessels in the path of that cervical vertebral or carotid artery. This can result in masking of the AVM angioarchitecture because of overlying cerebral vessels.
The Outreach DAC (Concentric Medical, Mountain View, California) is a 4.3F catheter designed to assist the Merci device (Concentric Medical) with clot retrieval in acute stroke.4 In the authors' opinion, the DAC provides triaxial support to improve stabilization of the microcatheter and a safer exit route for the microcatheter, without adding significant complexity to the procedure, compared with embolization with a guide and microcatheter alone. We investigated its application as an adjunctive tool for embolization of intracranial AVMs.
Technique
Angiography is initiated with the patient under conscious sedation with common femoral artery access. Four-vessel cerebral angiography is performed with a 90-cm 6F Envoy catheter (Cordis, Miami Lakes, Florida) to assess AVM anatomy. The Envoy is advanced into the desired vertebral or carotid artery at the skull base and is placed under continuous flush. The patient is systemically heparinized (goal activated coagulation time, <250 seconds). A microcatheter (ie, 165-cm Marathon [ev3/Covidien Vascular Therapies]) is introduced through the 136-cm DAC and advanced over a Synchro-10 wire (Boston Scientific, Natick, Massachusetts). Both microcatheter and DAC are connected to a continuously heparinized and pressurized flush. As we advance the microcatheter into the desired feeding pedicles, the DAC is advanced over the microcatheter intracranially. We remove the microwire and microcatheter and proceed with intracranial high-frame-rate DSA to evaluate the AVM angioarchitecture without obstruction of normal overlying vessels, then, we create high-quality roadmaps of the pedicle of interest. The microcatheter and microwire are reintroduced through the DAC, giving greater stability for the advancement of the microcatheter into the feeding pedicles and AVM nidus. Superselective Wada testing is performed through the microcatheter before embolization. If no changes are seen on detailed neurologic examination, we proceed with embolization.
Once embolization is deemed complete, the microcatheter is removed. The microcatheter is withdrawn until all slack is removed and a tug is noticed on the Onyx cast. As the microcatheter is withdrawn, the DAC advances, resulting in a counterforce to aid microcatheter withdrawal from the Onyx cast. Straightening of vessels occurs between the DAC tip and the microcatheter in the Onyx cast, aiding in removal of the microcatheter, in our opinion.
Final postembolization runs are obtained, the DAC and guide are withdrawn, the sheath removed, and the femoral access site is secured via a closure device.
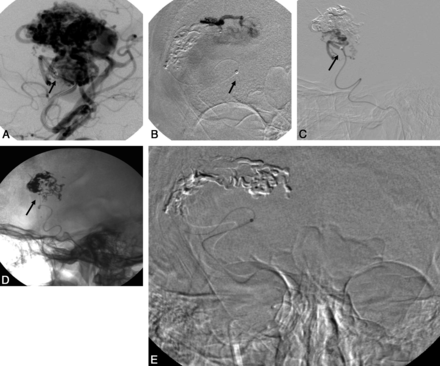
A case from our series is illustrated in Figs 1 and 2.
A 19-year-old man presented to the emergency department with a new-onset seizure. Initial noncontrast cranial CT scan revealed a 5-cm heterogeneous right frontal mass, suspicious for AVM (not shown). The patient received diphenylhydantoin (Dilantin) and was taken for DSA. Anteroposterior (A) and lateral (B) views of the 5 × 4 cm Spetzler-Martin grade 3 AVM demonstrate filling from the right middle cerebral artery.
Macro- (A) and microangiographic (B) runs with the microcatheter and DAC in place. The DAC is within a distal M3 branch (arrows). Lateral DSA (C) and lateral radiograph (D). After embolization, on withdrawing the microcatheter, the DAC is seen advancing into an M4 branch (arrows) and serves as a fulcrum for removing the microcatheter. E, Once the microcatheter is freed from the AVM nidus after the application of careful traction, the DAC and microcatheter can be seen as they are dislodged and snap back into the original location.
Discussion
Microcatheter delivery and stability are important when catheterizing arterial pedicles arising from distal tortuous vessels. The DAC provides microcatheter support for accessing these vessels by decreasing the length of microcatheter exposed to the ovalization forces within the tortuosity at the skull base. Because of its size (4.3F), more distal and selective angiographic runs are possible near the pedicle of interest for roadmapping and microcatheter navigation, allowing a more detailed appreciation of AVM angioarchitecture than possible from cervical guide runs.
Microcatheter disruption resulting from increased pressure from obstructed outflow may cause spillage of embolic materials from a more proximal location, such as anywhere from the cervical guide location to the AVM pedicle. Although not encountered in our patients, theoretically, the DAC, due to its distal intracranial location, may afford a layer of protection for embolic material spilling into the parent artery and direct it into the superselective vessel where the DAC is located.
Catheter entrapment is a known pitfall of AVM embolization with Onyx. Hauck et al5 reported catheter entrapment in 4.9% of their AVM embolizations with Onyx. This has been addressed to some extent by the use of a new microcatheter with a detachable tip6; however, this catheter is not available in the United States, and long-term associated outcomes are unknown. When the microcatheter is fixed, the DAC provides an ideal buttress to provide counterforce to withdraw the microcatheter. The DAC reduces the length of the microcatheter, which straightens out during this process, and advances, providing a fulcrum for safely removing the microcatheter without creating significant force or stretch on the microcatheter and/or affixed arteries. Compared with embolization without the DAC, in our opinion, there were reductions in the duration of the catheter-withdrawal process and in the pain experienced by the patients, who were awake and felt the straightening as a headache during catheter withdrawal.
The DAC is useful for endovascular embolization of most brain AVM pedicles. The limitations are few. Occasionally, the DAC may become occlusive in smaller distal vessels; this should be evaluated with DAC angiographic runs or puffs of contrast. Vessel occlusion was typically related to arterial size, rather than vasospasm around the DAC. An additional flush system is required for the DAC. This is an extra step but, in our opinion, worth the small amount of time expended.
Conclusions
The DAC is an intermediate-sized catheter that provides an effective means of guide-catheter and microcatheter support during AVM embolization. The DAC helps to provide a fulcrum for safely removing the microcatheter, allows specific runs of the pedicle of interest, and may afford a measure of protection in case of microcatheter rupture. The DAC can be used safely and effectively for AVM embolization and may be a helpful addition to the neurointerventionist's toolkit.
Acknowledgments
We thank Paul H. Dressel, BFA, for preparation of the illustrations and Debra J. Zimmer, AAS CMA-A, for editorial assistance.
Footnotes
-
Disclosures: Dr. Hopkins receives research support from Toshiba; serves as a consultant to Abbott, Boston Scientific, Cordis, Micrus, and W.L. Gore; holds a financial interest in AccessClosure, Boston Scientific, Claret Medical Inc., Micrus, and Valor Medical; has a board/trustee/officer position with AccessClosure, Claret Medical Inc., and Micrus (until September, 2010); belongs to the Abbott Vascular speakers' bureau; and receives honoraria from Bard, Boston Scientific, Cordis, Memorial Healthcare System, Complete Conference Management, SCAI, and Cleveland Clinic. Dr Levy receives research grant support (principal investigator: Stent-Assisted Recanalization in Acute Ischemic Stroke), other research support (devices), and honoraria from Boston Scientific and research support from Codman & Shurtleff Inc and ev3/Covidien Vascular Therapies; has ownership interests in Intratech Medical Ltd and Mynx/Access Closure; serves as a consultant on the board of Scientific Advisors to Codman & Shurtleff Inc; serves as a consultant per project and/or per hour for Codman & Shurtleff Inc, ev3/Covidien Vascular Therapies, and TheraSyn Sensors Inc; and receives fees for carotid stent training from Abbott Vascular and ev3/Covidien Vascular Therapies. Dr Levy receives no consulting salary arrangements. All consulting is per project and/or per hour. Dr Siddiqui has received research grants from the National Institutes of Health (coinvestigator: National Institute of Neurological Disorders and Stroke 1R01NS064592–01A1, Hemodynamic Induction of Pathologic Remodeling Leading to Intracranial Aneurysms) and the University at Buffalo (Research Development Award); holds financial interests in Hotspur, Intratech Medical, StimSox, and Valor Medical; serves as a consultant to Codman & Shurtleff Inc, Concentric Medical, ev3/Covidien Vascular Therapies, Guidepoint Global Consulting, and Penumbra; belongs to the Speakers' Bureaus of Codman & Shurtleff Inc and Genentech; serves on an advisory board for Codman & Shurtleff Inc; and has received honoraria from American Association of Neurological Surgeons' courses, an Emergency Medicine Conference, Genentech, Neocure Group LLC, and from Abbott Vascular and Codman & Shurtleff Inc for training other neurointerventionists in carotid stenting and for training physicians in endovascular stenting for aneurysms. Dr. Siddiqui receives no consulting salary arrangements. All consulting is per project and/or per hour. Drs Binning, Hauck, Orion, and Yashar have no financial relationships to disclose.
-
Funding for this study was received from NIH grants R01NS43924 and R01EB002873 and Toshiba Medical Systems Corporation.
-
The University at Buffalo Institutional Review Board granted approval for a retrospective review of patients' charts, electronic records, and imaging studies in accordance with the Health Insurance Portability and Accountability Act.
-
Unlabeled use of products: The use of the DAC (Concentric Medical) in the treatment of intracranial arteriovenous malformations is considered an off-label United States Food and Drug Administration application because this catheter has specifically been designed to work with the Merci retrieval system (Concentric Medical) to aid in clot removal.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- Received September 24, 2010.
- Accepted after revision December 26, 2010.
- © 2012 by American Journal of Neuroradiology