Abstract
SUMMARY: Polymorphous low-grade neuroepithelial tumors of the young (PLNTYs) are recently described CNS tumors. Classically, PLNTYs are epileptogenic and are a subtype of a heterogeneous group of low-grade neuroepithelial tumors that cause refractory epilepsy, such as angiocentric gliomas, oligodendrogliomas, gangliogliomas, and pleomorphic xanthoastrocytomas. Although they are a relatively new entity, a number of imaging and histologic characteristics of PLNTYs are already known. We present the imaging and pathologic findings of such a tumor as well as the surgical approach and clinical management.
ABBREVIATIONS:
- LGNT
- low-grade neuroepithelial tumor
- PLNTY
- polymorphous low-grade neuroepithelial tumor of the young
A 44-year-old left-handed woman with a history of depression and bipolar 1 disorder presented to our institution for suicidal ideation. She reported that her depressive symptoms had substantially worsened during the past year, and she had recently developed intermittent episodes of emotional lability, with sudden feelings of irritability and rage. In addition, she reported approximately 5 recent episodes of extreme fatigue in which she would fall asleep and be unarousable for hours, as well as shorter-duration spells characterized by facial and body paresthesias. The patient was admitted for psychiatric care and underwent neuroimaging to rule out a structural cause for her symptoms.
Imaging
An initial noncontrast head CT showed a partially calcified intra-axial mass in the left temporal lobe (Fig 1). MR imaging confirmed the presence of the lesion, which demonstrated heterogeneous signal, intralesional calcifications, and cystic components. Faint T1 hyperintensity was seen within the tumor, while other areas demonstrated minimal enhancement. Punctate areas of minimal intralesional restricted diffusion were present. Focal areas of elevated relative CBV were noted within the tumor on DSC perfusion imaging. A mild associated mass effect was seen, with effacement of the adjacent left lateral ventricle and expansion of an overlying left temporal lobe gyrus. However, there was no midline shift or herniation.
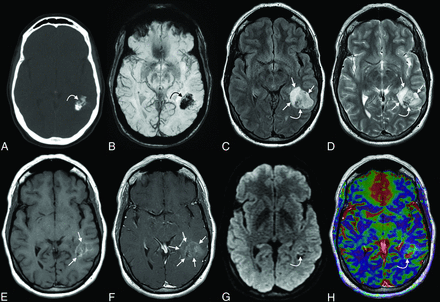
Initial CT and 1.5T MR imaging. Axial CT (A) image shows a sharply delineated mass within the left temporal lobe with dense intralesional calcifications, with blooming signal on the corresponding SWI (B) (curved arrows in A and B). Heterogeneous signal was noted within the mass on both FLAIR (C) and T2WI (D) (curved arrows on C and D). Some peripheral areas appeared cystic (straight arrows, C and D). No substantial surrounding vasogenic edema was observed. A mild associated mass effect was observed, including partial effacement of the left lateral ventricle. Faint T1-hyperintense signal was noted in the central components of the tumor (straight arrows, E), while a greater extent of the mass demonstrated mild enhancement (straight arrows, F). A few tiny foci of mildly restricted diffusion were observed centrally (curved arrow, G), which corresponded with low-intensity signal on ADC images (not shown), though these may have been artifactual because no high-grade features were seen on pathology. Some of the solid-appearing components demonstrated elevated relative CBV (curved arrow, H).
Preoperative imaging was completed on a 7T MR imaging scanner to optimize fMRI and DTI. On this examination, signal in the cystic areas was hypointense to CSF on T2-weighted images (Fig 2), which was thought to represent proteinaceous fluid within this region, particularly given its signal heterogeneity.
7T MR imaging demonstrates internal characteristics of the mass on T2WI. From left to right (A–C), heterogeneous solid material is seen centrally (long straight arrows), while cystic components are located peripherally (short straight arrows). Fluid within the cysts is heterogeneous, but typically hypointense to CSF. A mild associated mass effect is seen, including partial effacement of the left lateral ventricle (curved arrows).
Initial imaging raised concern for an oligodendroglioma, though the distinct tumor borders and dense central calcifications were thought to be atypical. A ganglioglioma and dysembryoplastic neuroepithelial tumor were also considered, though these are usually cortically based and have fainter internal calcifications.
Preoperative language fMRI was predominantly used to establish the patient’s hemispheric language dominance; because of the tumor location, left-hemispheric language dominance would have necessitated an awake resection with electrocortical stimulation mapping to prevent a postoperative language deficit. The patient performed 5 different fMRI language tasks in the scanner (Fig 3). The derived statistical maps demonstrated strong right-hemispheric language dominance for 3 expressive language–related activation sites (Broca area, dorsolateral prefrontal cortex, presupplementary motor area). Similarly, there was strong right-hemispheric language dominance for 3 receptive language sites (Wernicke area, Geschwind area, and visual word form area). The patient was consequently determined to be globally right-hemispheric-dominant for language, and intraoperative language mapping was unnecessary. Additionally, the patient’s right-hemispheric dominance suggested that the tumor may have been long-standing because its left-sided location may have prevented it from being symptomatic.
Presurgical mapping with fMRI and DTI performed on a 7T MR imaging scanner. Language fMRI (A–D) was accomplished using 5 different tasks: silent word generation (red), sentence completion (yellow), rhyming (cyan), reading comprehension (green), and semantic decision (magenta). In this left-handed patient, these tasks collectively demonstrated strong right-hemispheric lateralization of all 6 commonly identified language-activation sites, including the presupplementary motor area (Pre-SMA), language-related dorsolateral prefrontal cortex (DLPFC), Geschwind area (GA), Broca area (BA), Wernicke area (WA), and visual word form area (VWA).
Although marked intratumoral perfusion may result in arteriovenous shunting and hence confound fMRI blood oxygen level–dependent images, the intralesional relative CBV within this tumor was considered too minor to have such an effect. Thus, the determination of global right-hemisphere dominance was based solely on the fMRI findings.
The patient underwent gross total resection of the tumor approximately 3 months after presentation.
Operative Report
The patient underwent resection of the mass via a left temporal craniotomy. The surgeons elected to approach the tumor through a corticectomy of the left middle temporal gyrus, which was the shortest route to the tumor given the reassuring fMRI data. Intraoperative evaluation of the tumor demonstrated a hemorrhagic lesion. The tumor was ultimately removed in a piecemeal fashion. No obvious residual tumor was identified at the conclusion of the procedure. The patient did well postoperatively without immediate complication.
Pathology
Histologic analysis of the tumor demonstrated cells of variable size, with round-to-ovoid nuclei, as well as abundant coarse calcifications. Although the cells were similar to an oligodendroglioma, several distinguishing features were present (Fig 4). The nuclei of oligodendroglioma cells are typically more monotonous and rounder, and the focal microcalcifications seen are finer and much less extensive than those seen in the present case. More important, the tumor lacked isocitrate dehydrogenase 1/2 (IDH1/IDH2) mutations and codeletion of chromosomes 1p and 19q (see below), thereby precluding the diagnosis of an oligodendroglioma. High-grade features (microvascular proliferation, necrosis, and mitotic activity) were also absent.
Hematoxylin-eosin-stained photomicrographs show a prominent population of oligodendroglial cells with round-to-ovoid nuclei (arrow, A, scaled from 100×) as well as abundant calcifications, which appear as purple spherules of amorphous material (B, scaled from 100×). Immunohistochemical staining demonstrates uniformly positive nuclear OLIG2 expression (C, scaled from 100×), strong cytoplasmic expression of BRAF V600E (D, scaled from 200×), and widespread membranous expression of CD34 (E, scaled from 200×).
The next step in the histologic classification of this tumor was to differentiate it from other subtypes of low-grade neuroepithelial tumors (LGNTs), which can manifest a wide degree of histopathologic features. The abundant calcifications visualized are often seen in polymorphous low-grade neuroepithelial tumors of the young (PLNTYs). In addition, the tumor demonstrated strong CD34 expression and the diffuse positivity of oligodendrocyte transcription factor 2 (OLIG2) and B-Raf proto-oncogene, serine/threonine kinase (BRAF V600E), which are additional features of PLNTYs. Furthermore, characteristic histologic features of other LGNTs (eg, eosinophilic granular bodies, Rosenthal fibers, gemistocytes, specific glioneruonal element, ganglion cells) were absent.
Additional immunohistochemical studies showed no overexpression of p53 as well as retained ATRX chromatin remodeler (ATRX) expression. The Ki67 labeling index, a marker of proliferative activity, was minimal (<1%), supporting the diagnosis of a low-grade tumor.
A neuro-oncology-targeted next-generation sequencing panel examined 150 genes and confirmed the BRAF V600E mutation. No other sequence alterations of known pathologic significance were found; notably, there were no fibroblast growth factor receptor 2 or 3 (FGFR2 or FGFR3) fusions or IDH1 or IDH2 mutations. Chromosomal microarray showed no alterations of chromosomes 1p or 19q. Along with the lack of IDH1 or IDH2 mutation, the lack of 1p19q codeletion guides the differential diagnosis away from oligodendroglioma. BRAF V600E mutations are a recurrent genomic alteration described in PLNTYs (30% of tumors in the index series) and are mutually exclusive with FGFR fusions (identified in 40% of cases).1 Both BRAF and FGFR fusions cause downstream activation of the mitogen-activated protein (MAP) kinase pathway, and at present, the histology and subsequent behavior are similar in the presence of either alteration. Of note, BRAF alterations can be seen in other LGNTs, including gangliogliomas and pilocytic astrocytomas.
The diagnosis was PLNTY.
DISCUSSION
PLNTYs represent a nonependymal and non-neuronal subtype of LGNTs. The tumor has been recently recognized; it was first described by Huse et al,1 in 2017. The ages of affected patients within the original cohort were 4–32 years of age, with a median age of 17.5 years.1 Multiple subsequent case reports have described this tumor in patients in or near this age range.2-4 The sex of individuals in the index study was nearly evenly split (6 females, 4 males). As yet, PLNTY tumors remain unclassified by the World Health Organization. However, they are thought to be benign and appear to progress in a fashion similar to grade I tumors.5
Symptomatically, LGNTs often cause epilepsy that is early-onset and resistant to antiepileptogenic agents.3 These lesions have also been designated as long-term epilepsy–associated tumors.6-8 Gangliogliomas, dysembryoplastic neuroepithelial tumors, pleomorphic xanthoastrocytomas, and angiocentric gliomas are all subtypes of this growing list of such tumors. Nevertheless, like oligodendrogliomas, PLNTYs may also present with headaches or be found incidentally.5
Because it is a new and rare diagnostic entity, the imaging characteristics of PLNTY remain somewhat unknown. Johnson et al,4 in the largest imaging review conducted on such tumors, found them to be well-circumscribed, with heterogeneous intralesional signal. Most tumors have macroscopic calcification visible on CT. These calcifications are located centrally within the lesions, a distinct feature that may help radiologists distinguish them from other processes. The location of tumors, too, may be characteristic: PLNTYs are usually located in the temporal lobe, though lesions in the parietal, frontal, and occipital lobes have also been reported. Finally, cystic components were noted in 89% of cases, which were typically located peripherally (Fig 4).4
The index case fits these imaging characteristics neatly: A well-circumscribed temporal lobe mass with central calcifications, peripheral cysts, and heterogeneous internal signal should have raised suspicion of a PLNTY (Fig 5). An oligodendroglioma, too, may have demonstrated many of these imaging features, though the imaging findings overall favored a PLNTY (Table). Most notably, oligodendrogliomas have indistinct margins; sharp tumor borders are rarely seen in 1p19q-codeleted tumors.9 Oligodendrogliomas tend to have calcifications that are more typically gyriform/cortically-based.10 Extremely dense calcifications can also be seen in low-grade astrocytomas, albeit rarely.11 Intralesional signal in oligodendrogliomas is nearly always equal on FLAIR and T2 images, as opposed to astrocytomas, though this has not yet been studied in PLNTYs.12 The patient’s age was somewhat atypical for a PLNTY tumor: Forty-four years of age is outside the range of the largest prior case reviews, though PLNTY tumors have been found in patients as old as 57 years.13 Oligodendrogliomas, in comparison, are more likely to be diagnosed in adults, with a peak between 40 and 60 years of age.14 Finally, it is not entirely clear whether the patient’s spells represented true seizures; her symptoms were nonfocal, and findings of electroencephalography were normal. It is possible that the patient had seizures that propagated along cortical/subcortical pathways that were occult on electroencephalographic monitoring. However, 2 of the 10 patients first diagnosed with a PLNTY by Huse et al1 had clinical presentations other than seizures: one with headaches and the other with visual disturbances. As with most CNS tumors, clinically, presentations are variable and do not always predict pathology.
An artist’s illustration of a prototypical PLNTY. The tumors are typically located in the temporal lobe, well-circumscribed, and made up of mixed solid tissue, central calcifications (dashed arrow), and peripherally located cysts (curved arrow). Mass effect, if present, is usually minimal (short straight arrows). Reprinted with permission of the Mayo Foundation for Medical Education and Research. All rights reserved.
Comparison of clinical, imaging, and histopathologic characteristics of PLNTYs and oligodendrogliomas
A discrepancy was noted between the imaging features and pathology: Intratumoral perfusion was elevated, though the tumor lacked hypervascularity on histologic analysis. The findings on perfusion imaging were not thought to be artifactual because inherent susceptibility tends to cause absence or substantial reduction in relative CBV.
The patient in the index case had an excellent postoperative course following her surgical resection. She had complete resolution of her paroxysmal neuropsychiatric symptoms and subjective improvement of her cognitive status. Her language function was completely preserved postoperatively. Seven of 8 patients in the cohort originally described by Huse et al1 were disease-free following gross total resection (mean follow-up, 50.6 months). Only 1 patient had breakthrough seizures and follow-up imaging findings that were considered possibly concerning for tumor recurrence at 36 months after her operation.1
Case Summary
Although scantly described, PLNTY tumors have multiple characteristic imaging features: well-circumscribed masses, typically in the temporal lobe, with admixed heterogeneous solid and densely calcific material centrally and cysts peripherally.
Differential considerations include the following: oligodendroglioma, calcified low-grade astrocytoma, dysembryoplastic neuroepithelial tumor, pleomorphic xanthoastrocytoma, and ganglioglioma.
PLNTYs remain unclassified by the World Health Organization but appear to progress in a manner similar to grade I tumors.
Histologically, PLNTYs typically show calcifications and oligodendroglioma-like elements, though additional histologic elements can be present in variable amounts. Mutually exclusive BRAF mutations or FGFR2/3 fusions are common, and both activate the MAP kinase pathway.
Here, fMRI allowed a safe approach to the patient’s tumor without intraoperative language mapping.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- Received December 5, 2019.
- Accepted after revision February 13, 2020.
- © 2020 by American Journal of Neuroradiology