Abstract
SUMMARY: We observed a lesion pattern in a series of 4 cases of RANBP2-mutation–linked acute necrotizing encephalopathy, which appears to be specific for this condition. The setting of synchronous bilateral mammillary, amygdaloid, and lateral geniculate lesions, along with claustro-parahippocampal lesions, can serve as a diagnostic tool in this condition. We add several further details to the MR imaging features of the typical brain lesions encountered in this disease.
ABBREVIATIONS:
- ANE1
- acute necrotizing encephalopathy type 1
- RANBP2
- RAN binding protein 2
As a subgroup of the more heterogeneous entity named acute necrotizing encephalopathy of childhood,1 acute necrotizing encephalopathy type 1 (ANE1) is an underrecognized, infection-induced condition with autosomal dominant inheritance of several pathogenic mutations of the gene RANBP2, showing incomplete penetrance.2⇓-4 This condition is also named autosomal dominant acute necrotizing encephalopathy or, less commonly, infection-induced/triggered acute encephalopathy type 3. The gene encodes RAN binding protein 2 (RANBP2), a nuclear pore protein with many intracellular functions.5 Its dysfunction causes alterations in intracellular metabolism, mitochondrial distribution, nucleocytoplasmatic trafficking,5 and cytokine overproduction.6 On the basis of current scientific data, it is not clear how the pathophysiologic alterations predispose to attacks5 or why certain brain areas are specifically targeted.
MR imaging manifestations of encephalopathy and myelopathy are described in the literature.3,5,7⇓-9
MATERIALS AND METHODS
We registered 4 pediatric cases of RANBP2-mutation–linked acute necrotizing encephalopathy. Patient 1 is male and was 4 months old at the time of the first of his 2 attacks. Patient 2 is male and was 6 years old at his first and single attack. Patient 3 is female and was 10 months old at the first of 3 attacks. Patient 4 is female, and was 4 years old at the first attack, and had 2 attacks. Patients 1 and 2 are related (half-siblings). All our patients were treated in the intensive care unit during all their attacks. Clinical follow-up was 6–18 years: Patient 1 has mild cerebral palsy, well-controlled epilepsy, and only minor school problems. Patient 2 has no neurocognitive sequelae and maintains excellent grades at school. Patients 3 and 4 have minor motor coordination issues and moderate mental retardation based on neurocognitive testing.
We used 3T Achieva and 3T Ingenia (Philips Healthcare) scanners for our work. We performed our standard head imaging protocol during the attacks (axial DWI/ADC, T2, FLAIR, SWI, 3D TOF-MRA, non-enhanced, and contrast-enhanced 3D T1 sequences), and an additional 3D FLAIR sequence for assessing small necrotic lesions in the chronic phase.
RESULTS
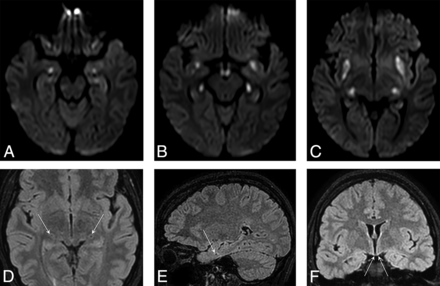
In the acute phase, restricted diffusion according to DWI and ADC map images, increased signal intensity on T2, FLAIR, and moderately decreased signal intensity on T1 were, in each case, seen in the bilateral mammillary, amygdaloid, and lateral geniculate bodies (patients 1, 2 and 4; Fig 1). Swelling of the mammillary bodies was also visible.
Axial DWI shows symmetric diffusion restriction in the amygdalae (A), at the anterior aspect of parahippocampal gyri, in the mammillary bodies (B), in the lateral geniculate bodies (B and C) and claustra (C). The 3D FLAIR images show the necrotic, lacunar lesions of specifically targeted, tiny structures, the lateral geniculate bodies (D), amygdala (E), and the necrotic atrophy of mammillary bodies (F).
In the chronic phase, necrosis isointense with CSF was evident in each case in the amygdalae, lateral geniculate bodies, and bilateral claustro-parahippocampal regions (Fig 1). Additionally, atrophy of the mammillary bodies was also detected (3D FLAIR, Fig 1).
Moreover, typical thalamic and/or brainstem (pontine, bulbar) lesions were observed.
Many authors refer to bilateral temporomedial involvement, but our current investigation shows that it is more precisely located at the most anterior, subcortical aspect of the parahippocampal gyri (Fig 1). These lesions were confluent with those in the claustrum (hence the above term “claustro-parahippocampal”) during the acute phase but became isolated lacunar necrotic lesions in the chronic stage.
The lesions were exceptional in targeting a specific area of white matter, while being similar in appearance to the other bilateral lesions targeting gray matter.
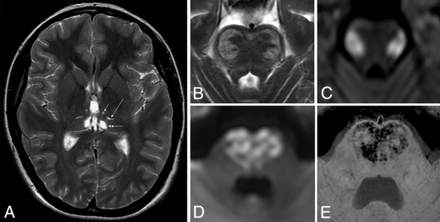
We found that the thalamic lesions were localized in the ventromedial and/or dorsomedial and/or dorsolateral (also named ventral posterior) nuclei. This was clearly demonstrated in the chronic, postnecrotic stage (Fig 2). In the acute phase, the perifocal edema may be more widespread (involving the whole thalamus or even the surrounding regions like the internal capsule).
Axial T2WI shows chronic thalamic lesions, identified by necrosis in the ventromedial (short arrow), dorsolateral (long arrow), and dorsomedial (double arrow) nuclei (A). The axial T2WI (B) and DWI (C) show typical lateral pontine manifestations with diffusion restriction and T2-hyperintensity during the acute phase. The axial DWI (D) and SWI (E) show medial pontine manifestations with diffusion restriction and petechial hemorrhage (heart-shaped lesion).
We found that the core of the pontine lesions in 3 of our patients arose at the lateral and, in 1 patient (during a different attack), at the central parts of the pontine base (Fig 2). One patient did not present pontine lesions. When the bilateral central parts were involved, they had a heart-shaped appearance on transverse sections (Fig 2).
Finally, we observed that typical bulbar lesions were located symmetrically in the regions of the vestibular nuclei.
DISCUSSION
To our best knowledge, we are the first to describe bilateral symmetric lesions of the lateral geniculate bodies in acute necrotizing encephalopathy type 1.
Bilateral lesions of these structures are demonstrated by MR imaging exclusively in the setting of another condition in literature.10 In this case of pancreatitis and microangiopathy, the rest of the associated lesions typically found in ANE1 were absent.
The lateral geniculate body is an anatomic part of the thalamus (located inferolateral to the thalamic pulvinar). Therefore, we emphasize that the typical thalamic lesions considered to be most common in the radiologic setting of this disease11 are located in other parts of the thalami, as described in the Results section.
We clarified that the symmetric lesions previously described in the temporal medial regions are actually situated at the most anterior subcortical aspect of the parahippocampal gyri and are unique in involving the white matter. Attention must be paid to differentiate them from the amygdaloid lesions because the proximity of the 2 small structures can lead to misinterpretation, appearing confusingly in figure captions of otherwise excellent articles.3
The “heart appearance” of the pontine lesions on MR imaging was previously described in a case of bilateral medial pontine infarction.12 We present ours as a variation of this radiologic sign in type 1 acute necrotizing encephalopathy.
CONCLUSIONS
On the basis of the analysis of the MR imaging studies of 4 patients, we describe several observations that can improve the diagnostic accuracy in ANE1 and may help the understanding of the pathomechanisms of this disease. The pattern of a synchronous appearance of bilateral amygdaloid, mammillary, lateral geniculate, and claustro-parahippocampal lesions appears to be constant and is potentially highly specific for this disease. This pattern, in combination with the features of thalamic and brainstem lesions described in detail, might play a role in further development of the neuroradiologic diagnostics or prognostic scoring. To estimate the specificity and sensitivity rates, further investigation on a broader patient group is needed.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Paper previously presented, in part, at: Annual Congress of the Hungarian Society of Neuroradiology, November 8–10, 2018, Eger, Hungary.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- Received May 17, 2021.
- Accepted after revision August 3, 2021.
- © 2021 by American Journal of Neuroradiology