Abstract
BACKGROUND AND PURPOSE: CTA has shown limited accuracy and reliability in distinguishing tandem occlusions and pseudo-occlusions on initial acute stroke imaging. The utility of early and delayed contrast-enhanced MRA in this setting is unknown. We aimed to assess the accuracy and reliability of early and delayed contrast-enhanced MRA for carotid bulb patency in patients with acute ischemic stroke.
MATERIALS AND METHODS: We retrospectively reviewed patients who had ICA occlusion and underwent thrombectomy with preprocedural early and delayed contrast-enhanced MRA in a single comprehensive stroke center. During 2 sessions, 10 raters independently assessed 32 cases with early contrast-enhanced MRA (with an additional delayed contrast-enhanced MRA sequence during the second reading session). Their judgments were compared with DSA as a reference standard. Accuracy and interrater agreement were measured. Five raters undertook a third reading session to assess intrarater agreement.
RESULTS: Accuracy for the assessment of carotid bulb patency with early contrast-enhanced MRA was limited (69%; 95% CI, 59%–79%), with moderate interrater agreement (κ = 0.42; 95% CI, 0.27–0.55). The second reading with an additional delayed contrast-enhanced MRA sequence improved both accuracy (82%; 95% CI, 73%–91%; P < .001) (raters corrected 43%–77% of incorrect diagnoses with early contrast-enhanced MRA alone; mean = 59%) and interrater agreement (κ = 0.56; 95% CI, 0.41–0.73; P = .07). Intrarater agreement was almost perfect, substantial, and moderate for 3, 1, and 1 raters.
CONCLUSIONS: Early contrast-enhanced MRA has limited accuracy and repeatability for the evaluation of carotid bulb patency in acute ischemic stroke. The additional delayed contrast-enhanced MRA sequence may improve accuracy and reliability.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- CE
- contrast-enhanced
- PO
- pseudo-occlusion
Several trials have demonstrated the benefit of mechanical thrombectomy in acute ischemic stroke (AIS) with anterior circulation large-vessel occlusion1 depicted by noninvasive intracranial vascular imaging (CTA or MRA). However, in case of tandem occlusion (ie, an ICA occlusion with an intracranial large-vessel occlusion), there remains uncertainty regarding the optimal management of the carotid bulb lesion,2 and the benefits of acute angioplasty/stent placement are controversial.3,4 Randomized controlled trials are needed to determine the best strategy and will thus require an accurate and repeatable noninvasive imaging method to select patients with an intracranial large-vessel occlusion and an additional carotid bulb occlusion,5 which will typically appear as an absence of visualization of the whole symptomatic carotid artery from the bulb.
However, in AIS, a single, large clot located above the ICA bulb can impede contrast ascension from the common carotid artery, leading to a false image of bulbar occlusion, an entity called ICA pseudo-occlusion (PO).6⇓⇓-9 It can, thus, be challenging to distinguish tandem occlusions and POs on initial acute stroke imaging. A previous study has shown limited accuracy and reliability of CTA for this task.10 CTA might thus be limited for the detection of tandem occlusions, whether in clinical routine (for endovascular management planning) or in research settings (for patient selection in a randomized controlled trial).
MR imaging offers several interesting features for acute stroke imaging and decision-making, including a high sensitivity for the detection of early ischemic lesions11 and the ability to detect stroke mimics12 and identify patients who will benefit from reperfusion therapy in case of unknown onset stroke.13 Noninvasive vascular imaging can then be performed with MRA: The TOF sequence is usually performed for the depiction of intracranial large-vessel occlusion, and early contrast-enhanced MRA (CE-MRA), for the assessment of the complete supra-aortic vasculature. As with CTA, cases with an intraluminal filling defect of the bulb on ICA on the stroke side might correspond to either a tandem occlusion or a PO. Delayed acquisition after gadolinium injection (delayed CE-MRA) might overcome this issue, but CE-MRA performance has not been thoroughly studied in this setting.
In this study, we aimed to assess the accuracy and reliability of early and delayed CE-MRA for the assessment of carotid bulb patency in patients with AIS.
MATERIALS AND METHODS
The local ethics committee (Centre Hospitalo-Univertaire Henri Mondor, Créteil) approved the study protocol and waived written informed consent. This study was prepared in accordance with the Standards for Reporting of Diagnostic Accuracy studies14 and the Guidelines for Reporting Reliability and Agreement Studies.15
Patients
We reviewed a prospectively collected endovascular data base in a single comprehensive stroke center between September 2016 and April 2018. We identified patients who underwent endovascular thrombectomy for AIS of the anterior circulation and in whom DSA showed occlusion of the ICA (at any level) on the symptomatic side. We excluded patients with other occlusion sites (such as isolated middle cerebral artery or posterior circulation occlusions) and patients who did not undergo MR imaging with early and additional delayed CE-MRA. The number of cases in the present study (n = 32) was superior to the recommendations of Donner and Rotondi16 for agreement studies.
Reference Standard
DSA was the reference standard for this study, similar to that in a previous study.10 Two interventional neuroradiologists (with 7 and 10 years of experience in neuroradiology, respectively) reviewed, in consensus, the DSA images obtained during thrombectomy, along with the patient's follow-up imaging and the report from the interventional neuroradiologist who performed the procedure, to determine the location of the occlusion.
DSA was performed with a biplane angiographic system (Axiom Artis dBA; Siemens). Carotid bulb occlusion on DSA was defined as an angiographically viewed obstacle that hindered the passage of contrast agent and/or the guidewire or catheter and the lack of backfilling of the carotid bulb during microcatheter angiography.9
In case of suprabulbar occlusion, 2 interventional neuroradiologists indicated the exact occlusion site based on the classification of Gibo et al,17 dividing the ICA into 4 parts: the cervical, petrous, cavernous, and intracranial portions.
Pseudo-Occlusion
ICA PO was defined as a unilateral intraluminal filling defect from the carotid bulb on the symptomatic side shown on early CE-MRA and in which DSA showed a suprabulbar occlusion.
MR Imaging
For all patients, MR imaging was performed at the same care center as thrombectomy. MRA examinations were performed at 3T (Magnetom Skyra; Siemens), including DWI, T2 FLAIR, SWI, and TOF-MRA.
When required, intravenous thrombolysis was directly initiated in the MR imaging scanner after these 4 sequences and before the acquisition of CE-MRA. Early CE-MRA was acquired after an intravenous injection of gadolinium-based contrast agent. A delayed CE-MRA acquisition was initiated immediately after the early CE-MRA sequence (without further contrast agent administration). This acquisition protocol resulted in fixed amount of time between gadolinium injection and CE-MRA acquisitions. Acquisition parameters of early and delayed CE-MRA are detailed in the Online Supplemental Data.
Raters
All raters were radiologists experienced in neurovascular imaging. Ten raters from 2 institutions, including 5 neuroradiologists and 5 interventional neuroradiologists with various levels of experience (detailed in the Online Supplemental Data) participated in the present study.
Readings
The MR imaging studies were first anonymized and uploaded in the PACS. Raters had no access to other imaging studies or clinical information other than sex, age, symptoms (ie, left or right motor deficit, aphasia), the initial NIHSS score, the time of symptom onset (when available), and the time of the brain MR imaging examination. Each rater performed 2 independent readings as follows: During the first reading, DWI, FLAIR, SWI, TOF-MRA and early CE-MRA were provided; during the second reading (performed 1 month later in a different order), an additional delayed CE-MRA sequence was provided for each patient.
For each case, raters indicated whether there was an occlusion of the ICA at the bulb level. The raters were blinded to the final diagnosis on DSA. Five raters (including 2 neuroradiologists and 3 interventional neuroradiologists) performed a third reading session (similar to the second one), at least 1 month later, to evaluate intrarater agreement.
Statistical Analysis
Mean [SD] was calculated for continuous variables, and frequency was reported for categoric variables. Sensitivity, specificity, and accuracy rates were calculated for each rater using 2 × 2 contingency tables. Group and subgroup validation parameters were calculated using mean sensitivity, specificity, and accuracy.
Interrater and intrarater agreement for the diagnosis of an ICA bulb occlusion was measured using Fleiss κ statistics with 95% bias-corrected and accelerated confidence intervals obtained by 10,000 bootstrap resampling.18 The κ comparison inference was conducted according to the method described by Vanbelle and Albert.19
All analyses were performed with R statistical and computing software, Version 3.3.2 (http://www.r-project.org/) and a significance level of 5%. Slight, fair, moderate, and substantial agreement categories were reported according to Landis and Koch.20
RESULTS
Patients
A total of 176 consecutive patients with AIS were treated by mechanical thrombectomy at our institution between September 2016 and April 2018. Among them, 102 patients with an isolated middle cerebral artery occlusion, 21 with vertebrobasilar artery occlusion, and 2 patients who did not receive endovascular treatment (because of catheterization failure) were excluded. Among the 51 remaining patients, 9 patients underwent CTA and 10 patients a early without delayed-phase CE-MRA were also excluded. The remaining 32 patients were thus included in the present study.
Patients' clinical, radiologic, and treatment characteristics are presented in the Online Supplemental Data. On the basis of DSA (reference standard), among the 32 patients, 6 patients had an isolated carotid bulb occlusion and 26 had a suprabulbar occlusion (5 with cervical, 2 with petrous, 3 with cavernous, and 16 with terminus occlusions). Among these same 26 patients, 8 patients had a radiologic pattern of PO (defined as a unilateral intraluminal filling defect from the carotid bulb on the symptomatic side shown with early CE-MRA, while DSA showed a patent bulb with a suprabulbar occlusion).
Illustrative cases of PO on CE-MRA and DSA are shown in Figs 1 and 2. Additional cases are shown in the Online Supplemental Data.
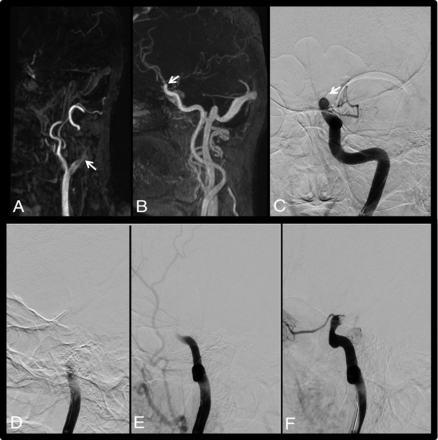
Case 10. The patient presented 2 hours after acute onset of right-sided hemiparesis and aphasia (NIHSS score of 24). Early CE-MRA (A) shows an intraluminal filling defect of the left proximal bulb ICA. Delayed CE-MRA (B) shows opacification of the left carotid artery up to the terminal ICA. DSA with selective catheterization of the left ICA confirms distal occlusion of the ICA (C, frontal DSA) with slow progression of iodine contrast (PO) (D–F, lateral DSA). White arrows indicates the occlusion site.
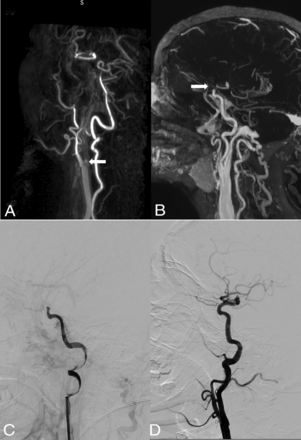
Case 19. This patient with unknown stroke onset presented with left-side hemiplegia and aphasia (NIHSS score of 20). Early CE-MRA (A) shows an intraluminal filling defect of the right proximal bulb ICA. Delayed CE-MRA (B) shows opacification of the right carotid artery up to the terminal ICA. DSA confirms distal occlusion of the right ICA (C, lateral DSA) and the absence of critical stenosis at the proximal ICA (D, lateral DSA). White arrows indicates the occlusion site.
Accuracy Study Results for the Assessment of Internal Carotid Bulb Patency
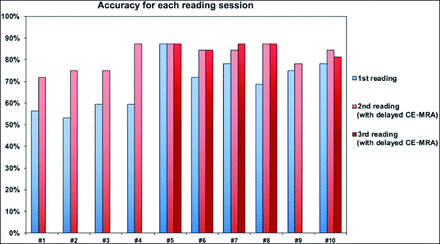
The assessment of internal carotid bulb patency using early CE-MRA alone (first reading) or with additional delayed CE-MRA (second reading) for all 10 raters is graphically displayed in the Online Supplemental Data. Accuracy parameters (sensitivity, specificity, and accuracy) are detailed in the Table. Graphic representations of the accuracy of each rater for the first, second, and third reading sessions are shown in Fig 3.
Graphic representation of the accuracy parameters of each rater for the assessment of internal carotid bulb patency for the first, second, and third reading sessions.
Diagnostic accuracy parameters and interrater agreement for the presence of carotid bulb occlusiona
During the first reading session, raters' accuracy for the diagnosis of carotid bulb occlusion ranged between 59% and 79% (mean accuracy, 69%; 95% CI, 59%–79%). Sensitivity rates ranged between 33% and 83% (mean sensitivity, 70%; 95% CI, 33%–83%), whereas specificity rates ranged between 50% and 92% (mean specificity, 68%; 95% CI, 50%–92%). No rater achieved 100% sensitivity, ie, no rater correctly identified all patients with a carotid bulb occlusion. The number of erroneous diagnoses (compared with DSA as the reference standard) ranged between 12.5% and 47% among raters (mean, 31%).
The second reading session (with access to an additional delayed CE-MRA sequence) improved accuracy for 9/10 raters (90%), now ranging between 73% and 91% (mean accuracy, 82%; 95% CI, 73%–91%; P < .001). For the last rater (rater 5), accuracy was identical during the first and second readings. Sensitivity was improved for 6/10 raters (including 5 raters with 100% sensitivity) and ranged between 67% and 100% (mean sensitivity, 87%; 95% CI, 67%–100%). Specificity was improved for 7/10 raters and ranged between 65% and 88% (mean specificity, 80%; 95% CI, 65%–88%). During this second reading session with access to delayed CE-MRA, raters corrected 43%–77% of incorrect diagnoses from the previous reading session (mean, 59%), while they erroneously modified 0%–20% of previously correct diagnoses (mean, 8%). Despite the use of additional delayed CE-MRA, most raters (>5/10) continued to provide incorrect diagnoses in 6/32 cases (18.7%) exclusively, by misdiagnosing ICA PO as a bulb occlusion: In 3/6 cases, DSA revealed a preocclusive stenosis of the carotid bulb associated with an intracranial ICA occlusion. In the 3 remaining cases, the quality of delayed CE-MRA was poor due to movement artifacts.
Interrater Agreement
Interrater agreement regarding the diagnosis of carotid bulb patency on early CE-MRA is shown in the Table and Fig 4A. Interrater agreement was moderate for all raters (κ = 0.41; 95% CI, 0.27–0.55), without significant differences between junior and senior raters. In 7/32 cases (22%), all raters agreed on the presence/absence of a carotid bulb occlusion. Interrater agreement during the second reading session (with access to delayed CE-MRA) was improved (κ = 0.56; 95% CI, 0.41–0.73) (Fig 4A) (P = .07): In 17/32 cases (47%), all raters agreed on the presence/absence of a carotid bulb occlusion.
A, Graphic representation of interrater agreement among all raters for the assessment of internal carotid bulb patency with early CE-MRA (first reading session) and with additional delayed CE-MRA (second reading session) (Fleiss κ). B, Graphic representation of intrarater agreement for 5 raters between the second and third reading session (both with access to delayed CE-MRA) (Cohen κ). κ values >0.6 (dotted line) define a “substantial” agreement.
Intrarater Agreement
Intrarater agreement study results are shown in the Online Supplemental Data and Fig 4B. Intrarater agreement was at least substantial for 4/5 raters, including 2 raters with a perfect intrarater agreement (κ = 1.0). Compared with the second reading session, accuracy performance during the third reading session was unchanged for 3/5 raters, improved for 1 rater, and decreased for 1 rater (but still improved compared with the first reading session).
DISCUSSION
This is, to our knowledge, the first study addressing the diagnostic accuracy and reliability of CE-MRA for the assessment of carotid bulb patency in patients with acute ischemic stroke. Our study demonstrates a limited accuracy and agreement for the diagnosis of internal carotid bulb occlusion with early CE-MRA compared with the reference DSA. The addition of delayed CE-MRA to our comprehensive MR imaging protocol improved both accuracy and interrater agreement.
ICA PO is a relatively common entity in case of acute terminal ICA occlusion, occurring in about 11%–46% of cases.6⇓⇓-9 The prevalence of ICA PO in our cohort (according to the reference standard DSA) was 8/32 cases (25%), which is in line with results from previous studies.6⇓⇓-9 The lack of differentiation between a “true” bulb carotid occlusion and ICA PO could lead to several issues. First, it may alter the inclusion of patients in future trials focusing on the management of tandem occlusions (for example, the ongoing French multicenter prospective randomized Thrombectomy In TANdem Occlusion [TITAN] trial21), by falsely enrolling patients with an aspect of tandem occlusion on initial noninvasive imaging while showing only an ICA PO on DSA.
Conversely, trials excluding patients with a tandem occlusion might erroneously exclude patients with an isolated intracranial occlusion. This exclusion might have happened in previous mechanical thrombectomy trials: In the Solitaire with the Intention for Thrombectomy as PRIMary Endovascular Treatment (SWIFT PRIME) study,22 for example, patients with a suspicion of extracranial ICA occlusion on initial imaging were excluded. Some of these patients possibly having an ICA PO could explain why the proportion of intracranial ICA occlusions was low in this study (15%)22 compared with other mechanical thrombectomy trials with no such exclusion criterion (for example, 31% in the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke [ESCAPE] trial23).
Second, the misdiagnosis of a carotid bulb occlusion instead of an isolated intracranial ICA terminus occlusion might alter the preprocedural management of the patient. According to recent studies, carotid stent placement with antiplatelet therapy seems to be the most effective therapeutic approach for tandem occlusion.3,24,25 Moreover, the efficacy of tPA in case of tandem occlusion has been reported to be weak,26 and the association of antiplatelet and tPA in the early phase of AIS is known to increase the risk of hemorrhagic transformation with no additional benefit.27
Given these results, physicians could be tempted to give antithrombotic agents and withhold intravenous thrombolysis because of a suspicion of tandem occlusion while the patients might have a PO. Finally, it may also misguide the operator for procedural planning: Carotid bulb occlusion necessitates specific tools that are not necessary in case of intracranial ICA occlusion such as noncompliant balloons and stents. The optimal management of tandem occlusions for both drugs and devices is still currently unknown, but an accurate and repeatable diagnosis on noninvasive imaging will be necessary in the future to tailor the treatment strategy.
A previous study has shown limited reliability of CTA for the distinction between a tandem occlusion and a PO.10 To overcome this issue, several studies have tested the utility of delayed-phase CTA.7,28⇓-30 Similar to delayed CE-MRA, delayed-phase CTA (with multiphase CTA28,29 or 4D-CTA30) improved the accuracy over arterial phase–only acquisitions. However, regarding 4D-CTA, given the limited coverage length acquisition (about 20 mm in thin-thickness reconstruction modes30), only ICA PO with distal intracranial occlusion might be detected. Moreover, the time necessary for image reconstruction may also restrict its use.30 Finally, these techniques also increase overall patient x-ray exposure.31
Early CE-MRA could be an alternative imaging technique. However, in our study, it showed a limited accuracy and repeatability for the assessment of carotid bulb patency in patients with acute ischemic stroke. Additional delayed CE-MRA improved both the accuracy and interrater agreement and showed strong intrarater agreement. Moreover, delayed CE-MRA does not need further contrast agent administration and requires little extra time: In our protocol, imaging-acquisition delay for delayed CE-MRA was 43 seconds. Otherwise, a range of techniques, such as compressed sensing and/or denoising techniques, could further reduce this delay.32 Other advanced MR imaging sequences such as high-resolution vessel wall imaging have been reported to accurately differentiate true tandem occlusion and ICA PO.33 However, given the acquisition time (7 minutes 43 seconds in the study mentioned above33), this type of sequence does not seem appropriate in the context of acute ischemic stroke. The time spent to perform a brain MR imaging, including postcontrast MRA, is a critical factor for the implementation of this technique in the setting of acute ischemic stroke, which is an absolute emergency. As an example, 8 minutes were necessary for DWI, FLAIR, SWI, and 3D-TOF acquisitions in our protocol. Additional early and delayed CE-MRA requires only 2–3 minutes (including localize sequence acquisitions, bolus-tracking, and the acquisition of the 2 aforementioned sequences). Although this scan duration is slightly longer than CT, a previous study has shown that MR imaging does not significantly change decision-making or impact functional outcome.34
We recognize certain limitations to our study. Our study population included patients scanned with MR imaging, which is not the main imaging technique for the diagnosis of acute ischemic stroke in most centers. Therefore, our findings cannot be extrapolated to centers using CT as first-line imaging. The large number of raters with varying degrees of experience could impact the accuracy of results. However, all raters except one (with identical accuracy during the first and second readings) improved their accuracy with the access to delayed CE-MRA. Greater agreement might have been reached if the readers received standardized training before the reading sessions.
We also cannot exclude the probability that the improved accuracy and interrater agreement during the second reading could be due to a “training effect” from the first reading session; however, the results of each reading session were not disclosed to the readers, so they did not have any feedback to adjust their judgments. Moreover, the second reading session was performed in a different order and at least 1 month later. We also cannot exclude the possibility of cervical clot migration between MRA and DSA in patients treated with intravenous tPA infusion. However, given the low efficacy of tPA in case of tandem occlusion26 and the short time between MRA and DSA (all patients were managed in a mothership paradigm), we can speculate that this phenomenon is very unlikely to occur. We did not assess the accuracy and reliability of the identification of the underlying cause of each tandem occlusion (dissection, atheroma, other). The etiology might modify the endovascular management,35 and the ability of noninvasive imaging to differentiate each cause necessitates further studies.
CONCLUSIONS
Our study demonstrated a limited accuracy and repeatability for the assessment of carotid bulb patency with early CE-MRA compared with the reference DSA in the context of AIS. The addition of delayed CE-MRA to our comprehensive MR imaging protocol improved both accuracy and reliability in this setting.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- Received May 21, 2020.
- Accepted after revision December 21, 2020.
- © 2021 by American Journal of Neuroradiology