Graphical Abstract
SUMMARY:
Giant cell arteritis (GCA) is the most common primary large vessel systemic vasculitis in the Western World. Even though the involvement of scalp and intracranial vessels has received much attention in the neuroradiology literature, GCA, being a systemic vasculitis, can involve multiple other larger vessels including the aorta and its major head and neck branches. Herein, the authors present a pictorial review of the various cranial, extracranial, and orbital manifestations of GCA. An increased awareness of this entity may help with timely and accurate diagnosis, helping expedite therapy and preventing serious complications.
ABBREVIATIONS:
- ACR
- American College of Rheumatology
- AION
- anterior ischemic optic neuropathy
- ESR
- erythrocyte sedimentation rate
- EULAR
- European League Against Rheumatism
- GCA
- giant cell arteritis
- LV-GCA
- large vessel GCA
- PMR
- polymyalgia rheumatica
- TAB
- temporal artery biopsy
- VWI
- vessel wall imaging
- STA
- superficial temporal artery
Giant cell arteritis (GCA), categorized as a large vessel vasculitis under the 2012 Revised Chapel Hill Consensus Conference, is the most common primary systemic vasculitis in the Western World in people older than 50 years.1⇓⇓⇓–5 The peak incidence of GCA occurs in individuals in their eighth decade of life. GCA is more common in Scandinavians and North Americans of Scandinavian descent and more common in women (F:M = 2:1).4,6,7 The lifetime risk of developing GCA is about 1% for women and 0.5% for men, with an overall GCA incidence of 20 per 100,000 population in people older than 50 years.4,8 Patients may present with a wide range of symptoms, including headache (75%), jaw claudication (30%), swelling or tenderness along the temporal artery (50%), visual (15%) or neurologic (30%) symptoms.4,5 There is substantial overlap with polymyalgia rheumatica (PMR). Approximately 40%–60% of patients with GCA have PMR, while approximately 15%–20% of patients with PMR have GCA.8 The precise pathophysiology of GCA is not well-defined. Seasonal variations in GCA onset may reflect the role of environmental factors in genetically prone individuals. A genome-wide association study in 2017 showed a strong human leukocyte antigen class II association, besides identifying risk polymorphisms in genes encoding plasminogen and an isoform of the α subunit of collagen prolyl 4-hydroxylase, which are consistent with alterations in vascular remodeling in disease susceptibility.7,9
GCA has two broad, overlapping phenotypes. Patients with predominantly cranial GCA (also referred to as C-GCA) often show involvement of branches of the external carotid artery, such as superficial temporal, facial, or occipital artery. A recent ultrasound-based study noted that superficial temporal artery (STA) involvement was more common (76%), followed by facial (41%) and occipital (31%) arteries. On the other hand, patients with large vessel GCA (LV-GCA) are more likely to have aortic and upper extremity arterial involvement, with axillary arteries being most frequently involved.7,10 GCA may classified based on the previously outlined criteria by the American College of Rheumatology (ACR) in 1990, which largely relied on clinical, lab, and pathologic abnormalities for GCA diagnosis. However, these have been criticized for their poor sensitivity and exclusion of extracranial large vessel involvement.3,5,11 For example, Muratore et al11 noted that while 95% of patients with cranial GCA met at least 3 ACR criteria needed for diagnosis, only 39% of patients with LV-GCA satisfied at least 3 criteria. Similarly, temporal artery biopsy (TAB), which is considered the standard for diagnosis, has low sensitivity.5 The widespread use of noninvasive vascular imaging has further necessitated the need for revised criteria to reflect current practice.
The 2022 ACR/European League Against Rheumatism (EULAR) updated GCA classification criteria to include: positive TAB or temporal artery halo sign on ultrasound (+5), erythrocyte sedimentation rate (ESR) ≥50 mm/h or C reactive protein ≥10 mg/L (+3), sudden visual loss (+3), morning stiffness in shoulders or neck, jaw or tongue claudication, new temporal headache, scalp tenderness, temporal artery abnormality on examination, bilateral axillary involvement on imaging and fluorodeoxyglucose–positron emission tomography activity throughout the aorta (+2 each). A cumulative score of ≥6 points was shown to achieve a sensitivity of 87.0% (95% CI: 82.0%–91.0%) and specificity of 94.8% (AUC: 0.91; 95% CI: 0.88–0.94) for GCA diagnosis in the validation cohort (Table).12 Application of these criteria however, should only be considered once a diagnosis of vasculitis has been made and alternate diagnoses have been excluded.
Updated classification criteria for GCA diagnosis
Before the introduction of corticosteroid therapy, the estimated mortality rate among patients with GCA was approximately 12.5%. With appropriate treatment, however, the long-term outcomes and survival rates are similar to age-matched population.4 Important complications in GCA include vision loss (up to 15%), aortic aneurysms (10%–15%) and dissections, and cerebrovascular events (2%–4%).8,11,13
Typical histopathologic findings in GCA include vessel wall inflammation, intimal thickening, and internal elastic lamina fragmentation (Fig 1). Multinucleated giant cells are seen in only about one-half of the cases. Other findings include lympho-mononuclear predominant panarteritis and inflammation of the vasa vasorum.4,8 Presence of fibrinoid necrosis often implies alternate diagnosis such as antineutrophil cytoplasmic antibody-associated vasculitis.8
H&E (A) and Verhoeff-van Giesson (VVG) (B) stain photomicrographs from a temporal artery biopsy in a positive GCA case reveal severe arteritis with inflammatory lymphocytic cells throughout the vessel wall (A and B). There is loss of the internal elastic membrane (B, arrow) with marked intimal fibroplasia (asterisk), resulting in complete obliteration of lumen. Inserts with magnified views show multinucleated giant cells (arrowheads) interspersed between lymphocytes.
No single imaging technique is generally sufficient to evaluate disease extent and severity in GCA. For the same reason, the 2023 EULAR update on imaging recommendations suggests different imaging modalities for cranial and extracranial GCA. The guidelines also make suggestions for technical and operational parameters for the various imaging modalities, which may be useful for designing and implementing imaging protocols at the institutional level.14 Besides the recommendations, the paper also outlines 3 overarching principles: 1) performing early imaging in suspected GCA, which should not impede treatment initiation; 2) imaging by trained experts using standardized protocols; and 3) avoiding additional testing in patients with high clinical suspicion and positive initial imaging as well as patients with low clinical suspicion and negative imaging findings.14
Even though imaging in GCA has largely focused on the scalp and extracranial vessels, additional imaging findings in the orbits, temporalis muscle, and intracranial vessels have also been reported.3,5 Herein, we review the previously reported imaging findings in GCA, which can be helpful in accurate and timely detection of this systemic large vessel vasculitis.
VASCULAR FINDINGS IN CRANIAL GCA
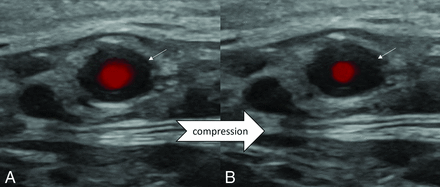
Most studies have broadly focused on the involvement of the superficial temporal artery branches, with some studies additionally evaluating intracranial and orbital vessels. As per the most recent EULAR recommendations, ultrasound of the temporal and axillary arteries is recommended as the first-line imaging technique in patients with suspected, predominantly cranial GCA, with a noncompressible halo sign being the most suggestive finding.14,15 Additional imaging findings may include vascular stenosis and/or occlusion. The halo sign refers to the presence of homogeneous, hypoechoic wall thickening, which is concentric on transverse images (Fig 2). The halo sign has a sensitivity of 68% and a specificity of 91% for GCA diagnosis. Its specificity reaches up to 100% when present bilaterally.3,4 A recent meta-analysis only using studies with low risk of bias noted a pooled sensitivity of 88% (95% CI: 82%–92%) and specificity of 96% (95% CI: 86%–99%) for ultrasound in GCA diagnosis.15 The halo sign may also be seen in other involved vessels.10
Halo sign and compressibility test in a patient with biopsy-proved GCA. Ultrasound images (A and B) show a dark hypoechoic halo around the STA lumen (arrows), representing the vessel wall inflammation with partial loss of normal compressibility and decrease in flow.
Ultrasound evaluation of the vessels should ideally include the common temporal arteries and their frontal and temporal branches, as well as the axillary arteries. This is preferably performed with linear probes with at least 15−18 MHz and ≥ 12−15 MHz frequencies for temporal and axillary arteries, respectively.3 Additional evaluation of facial, vertebral, occipital, subclavian, and femoral arteries may be helpful when the GCA diagnosis is not clear. Well-recognized advantages of ultrasound include easier availability, noninvasive nature, and lack of any radiation or need for intravenous contrast. The main limitation is operator dependence.3,16
Several recent studies have also evaluated the utility of high-resolution MRI vessel wall imaging (VWI) for cranial GCA, which is generally performed by using standard gadolinium dose contrast, with a T1-based sequence using fat-suppression and a 5-minute delay between contrast injection and image acquisition.1,5,13 The reported imaging findings in GCA cases include thickening and enhancement along the vessel walls of the superficial temporal artery and its branches, as well as the occipital artery. The enhancement is generally concentric and can be segmental (Fig 3).1,17,18 The enhancement may be semiquantitatively assessed based on the previously described 4-point scale by Klink et al,1 with scores of 0−1 representing physiologic features and scores of 2−3 reflecting vessel wall involvement. Siemonsen et al18 noted that most patients with GCA showed clear signs of mural inflammation in at least 2 affected vascular segments. Luminal stenosis and diffusion restriction along the involved vessel segments may also be seen (Fig 4).3,19 With treatment, changes of vasculitis improve, though residual wall thinning and pseudoaneurysm may be seen (Fig 5). The reported sensitivity and specificity of MRI-VWI on the more recent meta-analyses were 91% and 78%, respectively, when compared with temporal artery biopsy as the reference standard.5 MRI-VWI does have a high negative predictive value for cranial GCA, and a normal MRI-VWI study may imply a low probability of a positive TAB.20 The 2023 update to the EULAR guidelines recommends both high-resolution MRI and FDG-PET as alternatives to ultrasound for assessment of cranial arteries in suspected GCA.14
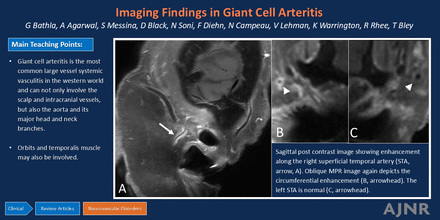
Sagittal postcontrast T1-SPACE image shows circumferential wall enhancement along the right superficial temporal artery (STA, arrow, A). Oblique MPR images perpendicular to the involved vessel (in A) show corresponding circumferential enhancement (arrowhead, B). The left STA (arrowhead, C) is normal.
Axial DWI in a treatment-naïve patient with GCA showing scattered foci of increased diffusion signal along bilateral scalp vessels (arrowheads). Inset in bottom left shows magnified diffusion-weighted signal changes along the right frontal STA branch.
Long-standing GCA in a 66-year-old woman with pseudoaneurysm of the superficial temporal artery. Frontal (A) and lateral (B) projections on catheter angiogram (External carotid artery injection) reveal multifocal areas of narrowing involving the frontal and parietal branches of STA (A and B, arrows). A small pseudoaneurysm is noted along the frontal branch of STA (A and B, arrowheads). Temporal artery biopsy with H&E (C) and Verhoeff-van Giesson (VVG) (D) stains reveals marked atrophy of the arterial wall (C and D, arrows), suggesting healing with pseudoaneurysm (C and D, arrowheads).
In general, MRI-VWI evaluation at higher magnet strength is more fruitful, and 3D-VWI sequences perform better in detecting GCA changes, being more specific when compared with 2D-VWI sequences (91% versus 84%), with similar overall sensitivity (70% versus 72%).5,17 3D-VWI sequences also have additional advantages of larger field-of-view, the ability to generate reformatted images without loss of image resolution, and evaluation of multiple vessels along their course.17
A cross-sectional study recently compared ultrasound and MRI for GCA in patients with both newly diagnosed and established disease. The authors noted that in this small patient cohort, ultrasound detected vasculitic changes more frequently than MRI (37% versus 21%, P < .001) and was also more sensitive in detecting vasculitic changes in larger head and neck vessels. However, the lack of vasculitic changes on MRI/MRA was significantly associated with disease remission. With ultrasound, vasculitic changes were noted in both active and inactive disease. Notably, the study used 1.5T MRI, and larger head and neck vessels (such as carotid, axillary, and subclavian vessels) were only assessed on MRA and not MRI.16
When compared with ultrasound, the main disadvantages of MRI-VWI include lack of wider availability, requirement of intravenous contrast, need for subspecialty expertise in image evaluation, and generally longer wait times. The latter is especially important as the imaging findings can quickly improve after high-dose corticosteroid administration, thus reducing diagnostic sensitivity.1,3,4 MRI-VWI is also more prone to artifacts from venous or slow flow and nonsuppression of intraluminal signal, which can be occasionally problematic (Fig 6). Finally, MR imaging may be contraindicated due to patient-specific factors (such as cochlear implants, aneurysm clips, and so forth).16
Axial postcontrast images reveal flow artifacts in the right occipital vein (arrows), which should not be misinterpreted as mural inflammatory changes. There is circumferential enhancement along the right superficial occipital artery (arrowheads).
However, MRI-VWI has an additional advantage of allowing simultaneous evaluation of orbits, temporalis muscles, and intracranial vessels.5 The latter may be affected in about 10%–15% (reported up to 50% in some studies) of patients with GCA, with intradural ICA and vertebral vessels most commonly involved. The involved vessels may show circumferential wall thickening and enhancement, similar to extracranial counterparts.13,18,21 A recent study noted that most lesions involve a vessel length greater than 5 mm, with none showing >70% luminal stenosis.13
There is limited literature on the role of cranial CT angiography in GCA. Conway et al22 previously retrospectively evaluated CTA head studies in a cohort of 14 treatment-naïve patients who were subsequently diagnosed with GCA, along with a similar number of age-matched controls. Blurred vessel margins and perivascular enhancement was noted in 10 cases and 2 controls, yielding an accuracy of about 78.6% for CTA (Fig 7). Interestingly, the presence of STA occlusion, stenosis, or calcification was not significantly different between the 2 groups.22
Sagittal CTA-MPR image (A) reveals blurring of vessel margins and subtle fat stranding along the frontal branch of the right STA. Volume-rendered image (B) shows scattered areas of vessel irregularity and stenosis along the STA branches (arrows).
More recently, studies using 18F FDG-PET have also shown promising results in cranial GCA diagnosis (Fig 8). Nielsen et al23 studied a cohort of 44 patients with an equal number of age-matched controls. Based on presence or absence of FDG uptake in the temporal, maxillary, and vertebral arteries, PET-CT had 82% sensitivity and 100% specificity.23 Limitations of FDG-PET include restricted availability, high cost, and radiation.3
Axial CTA image (A) in a patient with newly diagnosed GCA shows reduced contrast opacification along the left STA (arrow) and a normal-appearing right STA (arrowhead). Fused PET-CT image (B) from the same patient at a slightly cranial level shows prominent radiotracer uptake on the left (circled).
Vascular Findings in Large Vessel GCA
In comparison with cranial GCA, LV-GCA predominantly involves the thoracic aorta and aortic arch branches, with or without cranial vessel involvement.3,24 Kermani et al,25 in their prospective, longitudinal, multicenter study, noted that 66% of patients with GCA had at least 1 large vessel arterial lesion at diagnosis and 39% of those with follow-up imaging developed new lesions, often in the first 2 years. All patients with new lesions had baseline imaging abnormalities.25 Patients with LV-GCA tend to behave slightly differently than patients with cranial GCA, are younger at presentation, have longer symptom duration before diagnosis, are more likely to have associated polymyalgia rheumatica and higher incidence of relapses, and require longer corticosteroid treatment. These patients often have fewer cranial symptoms and vision loss and are generally more TAB-negative.11,24
Frequently involved vessels include aorta, subclavian, axillary, and brachial arteries, with involvement of lower extremity arteries being less common.11 The 2023 update to the EULAR guidelines recommends the use of FDG-PET as the preferred technique for evaluation of extracranial arteries, with a sensitivity of 76% and specificity of 95% with the clinical criteria as the reference standard. There is overall limited evidence on the utility of CTA and MRA in LV-GCA.14
On FDG-PET, areas of active vasculitis show increased radiotracer uptake along the vessel wall/course (Online Supplemental Data). Besides evaluating the presence or absence of inflammation, FDG-PET is also helpful to determine overall extent of vasculitis and simultaneously exclude presence of underlying malignancy and infection. Like MRI, the diagnostic accuracy can drop considerably in treated patients; therefore, imaging very early in the disease course is essential.3,4,14
CTA and MRA can also be used to evaluate LV-GCA and demonstrate wall thickening, enhancement, and long segment tapering stenosis along upper extremity vessels, the latter being present in 3−15% of cases (Online Supplemental Data).4,8 Underlying aortitis most commonly involves the thoracic aorta and may be clinically silent.4 Espitia et al26 noted aortic complications in 23.5% of their patients with LV-GCA, predominantly consisting of aneurysms and dissections. These were seen after a median delay of 27 months postdiagnosis and were significantly more common in patients with symptomatic aortitis, defined as presence of dorsal/lumbar/abdominal pain and/or aortic insufficiency.26 Quinn et al27 compared FDG-PET and MRA in LV vasculitis (including GCA and Takayasu arteritis) and noted that MRA outperformed FDG-PET for evaluating disease extent but had lower interreader correlation. Even though clinical status was more closely correlated with FDG-PET activity, about 51% of patients with LV vasculitis in clinical remission had active disease by both MRA and FDG-PET.
Orbital Findings in GCA
Vision loss remains one of the most dreaded complications of GCA, often occurring suddenly and painlessly. It may be unilateral or bilateral, with a higher risk of bilateral involvement if the unilateral vision loss is not emergently treated with high-dose corticosteroids.4,8
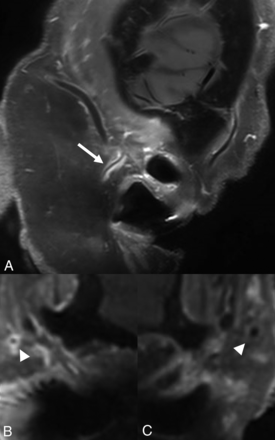
Inflammatory involvement of intraorbital structures has been reported in about one-third of patients with GCA, most commonly along the optic nerve sheath followed by the ophthalmic artery and intraconal fat (Fig 9).28 Gospe et al29 also noted that both intracranial ICA and optic nerve sheath enhancement were observed in patients with TAB+ GCA and a combination of these two imaging findings was highly specific for GCA. Similarly, Sommer et al30 noted that MRI-VWI showed bilateral orbital involvement in 50% of cases with arteritic anterior ischemic optic neuropathy (AION) when only unilateral corresponding changes were noted at fundoscopy, suggesting improved detection of subclinical disease and patients at risk of further vision loss. Another study noted that inflammatory changes along the ophthalmic artery were present in all cases with arteritic AION but in none with nonarteritic AION.31 Finally, Remond et al32 noted that all patients with GCA-AION showed optic nerve head enhancement (central bright spot sign). Similar findings were also noted in about 50% of patients with nonarteritic AION, while none of the healthy controls showed optic nerve head enhancement. The authors postulated that absence of central bright spot sign may suggest underlying nonarteritic AION.
Axial T1-SPACE postcontrast image through the orbits in a patient with newly diagnosed GCA shows bilateral retrobulbar intraconal enhancement near the apex with involvement of bilateral ophthalmic arteries (arrows).
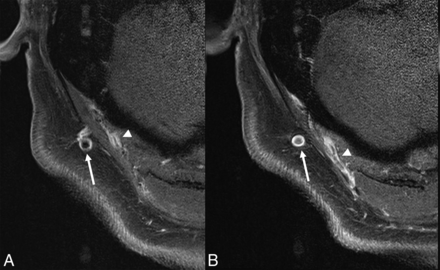
Additional findings in patients with GCA, as described on MRI, include temporalis muscle inflammation (about 20%) and vasculitis of the deep temporal artery (34%–49%), with simultaneous involvement of both structures reported in about 20% of patients (Fig 10). The latter shows a moderate correlation with jaw claudication.6
Axial T1-SPACE postcontrast image (same patient as Fig 9) shows asymmetrically increased enhancement along the right temporalis muscle (arrows) and along the deep temporal artery (arrowhead).
Challenges and Future Directions
A recent population-based cohort study noted that even though the incidence of GCA remained constant over the past 2 decades (1996−2018), the proportion of patients with GCA receiving TAB declined sharply from 70–80% to 29–39% after 2016, while the use of diagnostic imaging increased from 2% to 66% between 2000−2018, underscoring the role of noninvasive imaging in GCA diagnosis.33
Despite the increasing role of imaging in GCA diagnosis, its utility in follow-up remains a topic of intense research. Koster et al,24 for example, noted that there was a discordance between imaging findings and clinical symptoms, especially during follow-up. Another study noted that even though tocilizumab led to complete clinical and biochemical remission in their cohort, imaging abnormalities of the extracranial large arteries only normalized in one-third of the patients.34 On the other hand, treatment rapidly improves superficial cranial vessels and mural inflammatory changes such as intima-media thickness, contrast enhancement, and mural thickening.1,35 For these reasons, the added value of imaging in response assessment, to define remission, in predicting short and long-term outcomes and its association with novel laboratory markers remains under investigation.14 Similarly, the use of imaging findings as an outcome tool needs to be prospectively evaluated in randomized controlled trials.
Additionally, some recent studies have shown that concurrent evaluation of cranial and LV-GCA improves overall sensitivity without negatively impacting the specificity of GCA diagnosis with both ultrasound and PET-CT. The diagnostic accuracy of combined cranial and LV-GCA with MRI remains under investigation.36 Finally, some recent studies have shown promising results in terms of diagnosis and management of GCA by using artificial intelligence–based methods, with either imaging or nonimaging (patient) data.37,38 These, however, need to be prospectively validated on larger patient cohorts.
CONCLUSIONS
GCA can have a spectrum of imaging manifestations involving both cranial and extracranial sites. Imaging plays an increasingly important role in timely and accurate diagnosis. A broader recognition of its imaging abnormalities and awareness of its protean manifestations may help with prompt initiation of therapy and avoid serious complications.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- Received April 29, 2024.
- Accepted after revision June 13, 2024.
- © 2025 by American Journal of Neuroradiology