Abstract
BACKGROUND AND PURPOSE: Discrete focal lesions in the splenium of the corpus callosum on MR images in epileptic patients have received little attention in the literature. Our purpose was to describe these lesions, which may be related to the toxicity of antiepileptic drugs (AEDs), and to discuss the possible mechanisms of their development.
METHODS: We examined six patients with epilepsy whose brain MR imaging findings showed a discrete focal nonhemorrhagic lesion in the splenium of the corpus callosum. The medical records and MR images were reviewed retrospectively with respect to the patients' clinical history, medication, and laboratory findings to determine the etiology of the lesion.
RESULTS: In all six patients MR imaging showed a focal lesion in the splenium of the corpus callosum, which was ovoid in shape and 15 to 19 mm in size. In the three patients who received contrast material, there was no enhancement of the lesion. Four of six patients had a history of medication with dilantin, in two of whom the level of serum dilantin was found to be elevated (22.3 μg/mL and 70.4 μg/mL, respectively). Vigabatrin was administered in three patients, one of whom took dilantin together with vigabatrin. In two patients, the focal lesion in the corpus callosum disappeared on follow-up MR images after withdrawal of dilantin and/or vigabatrin.
CONCLUSION: A discrete, focal, ovoid, nonhemorrhagic lesion in the splenium of the corpus callosum may be seen on brain MR images of patients with epilepsy. The lesion is considered to be reversible demyelination related to AEDs toxicity.
We noted a discrete focal nonhemorrhagic lesion in the central portion of the splenium of the corpus callosum on the MR images of six patients with epilepsy. To our knowledge, there has been only one report documenting a focal lesion in the central portion of the splenium of the corpus callosum in association with epilepsy, the cause of which was speculated to be transient focal edema related to the transhemispheric connection with secondary generalized seizure activity (1). The possible mechanisms for the development of this lesion are discussed.
Methods
Between May 1994 and March 1998, approximately 1200 patients with epilepsy were examined with MR imaging. Among them, we identified six patients whose brain MR images showed a focal nonhemorrhagic lesion in the splenium of the corpus callosum. The patients consisted of three men and three women, ranging in age from 20 to 42 years (mean age, 31 years). The diagnosis included mesial temporal sclerosis (n = 4), dysembryoplastic neuroepithelial tumor (n = 1), and neurocysticercosis (n = 1). All six patients had complex partial seizures, one of whom had secondary generalized tonic-clonic seizures (case 6). The interval between the latest seizure and MR imaging was 3 to 4 weeks (n = 4) or 5 to 6 weeks (n = 2).
All MR studies were performed with a 1.5-T superconducting magnet with T1-weighted spin-echo (SE) (500/11 [TR/TE] sequences in axial and sagittal planes and proton density- and T2-weighted SE (2500/30,80) sequences in the axial plane. In five of the six patients, oblique coronal T2-weighted fast spin-echo (FSE) imaging of the hippocampus was performed for temporal lobe epilepsy, and two of these patients also had follow-up MR imaging. FSE imaging was performed with parameters of 6000/90, 3-mm-thick sections, 25-cm field of view, and 256 × 512 matrix. The angle of the imaging was perpendicular to the long axis of the hippocampus. In the patient with neurocysticercosis, MR imaging was performed twice, with a 1-month interval. Contrast-enhanced (0.1 mmol/kg gadopentetate dimeglumine) T1-weighted SE images were obtained in three patients.
Medical records were reviewed with regard to the patients' clinical history, medication, and laboratory findings to determine the etiology of the lesion in the corpus callosum.
Results
In all six patients, a discrete focal lesion was seen within the central portion of the splenium of the corpus callosum with high signal intensity on T2-weighted images and low signal intensity on T1-weighted images. The lesions were 15- to 19-mm × 9- to 11-mm in size and were nearly uniform and ovoid in shape (Figs 1–3⇓⇓). In the central area of this lesion, a small area of isointensity relative to the rest of the corpus callosum was seen on both T1- and T2-weighted images (Figs 1 and 2). There was no enhancement of the lesion on contrast-enhanced T1-weighted images obtained in three patients. No other lesions were found in the rest of the brain, including the fornices, the hypothalamus, the thalamus, or the cerebellum.
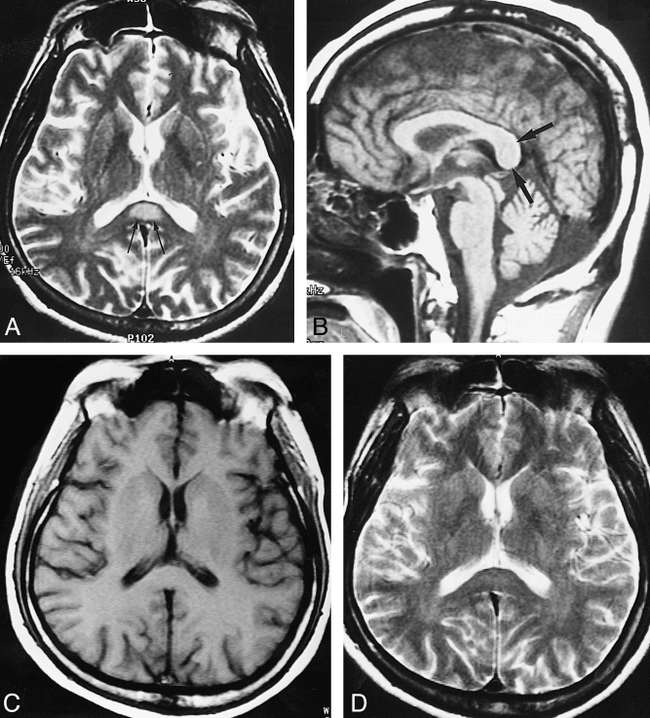
Case 1: 20-year-old woman with hippocampal sclerosis.
A, Left hippocampal head shows slight hyperintensity (arrows) relative to normal right hippocampal head on coronal T2-weighted FSE MR image.
B, Axial T2-weighted MR image shows a focal ovoid hyperintensity lesion (white arrows) in the splenium of the corpus callosum. There is a small, less hyperintense area (black arrow) within the lesion, presumably indicating a spared area of the white matter.
C, Sagittal T1-weighted MR image shows a well-defined focal low-signal-intensity lesion (solid arrows) containing a small, central isointense focus (open arrow), which probably corresponds to the less hyperintense area on the T2-weighted image (B).
D, Follow-up MR image, obtained 4 months after withdrawal of dilantin and vigabatrin, shows that the focal lesion in the corpus callosum has disappeared.
Case 6: 23-year-old woman with dysembryoplastic neuroepithelial tumor.
A, Coronal T2-weighted FSE MR image shows a hyperintense mass with no peritumoral edema (arrows).
B, No lesion is seen in the corpus callosum on initial T2-weighted MR image.
C, T2-weighted MR image obtained 7 days after initiation of vigabatrin shows a focal, ovoid, hyperintense lesion in the splenium of the corpus callosum (solid arrows). A central hypointense area (open arrow) is seen within the lesion, which probably indicates a spared part of the white matter.
Case 2: 42-year-old man with hippocampal sclerosis.
A and B, A focal lesion is seen within the central portion of the splenium of the corpus callosum on initial MR images in the period of dilantin intoxication (arrows).
C and D, The lesion disappeared on the follow-up MR images 6 months after discontinuation of dilantin.
According to the medical records, four of the six patients had a history of medication with dilantin. In two of the four patients, the level of serum dilantin was found to be elevated (22.3 μg/mL and 70.4 μg/mL, respectively), whereas the other two patients, whose serum dilantin level was not examined, had diplopia, dizziness, and/or gingival hypertrophy, which are known to be dilantin-related side effects. Vigabatrin was given as add-on therapy (1.0 to 1.5 g/day) in three patients, one of whom was taking dilantin simultaneously. The duration of vigabatrin medication in the three patients was 17 months (case 1), 2 months (case 5), and 7 days (case 6).
In two patients (cases 1 and 2), a focal lesion in the corpus callosum disappeared on the follow-up MR images obtained 4 months and 6 months, respectively, after withdrawal of dilantin and/or vigabatrin (Figs 1D and 3C and D). In the patient with neurocysticercosis (case 3), no focal lesion was seen in the corpus callosum on the initial MR study; however, on the follow-up examination, 20 days after he had taken dilantin for 10 days, a focal lesion appeared in the splenium of the corpus callosum. In the patient with a dysembryoplastic neuroepithelial tumor (case 6), no lesion was seen on the initial MR study obtained 3 years earlier, but a focal lesion in the splenium of the corpus callosum appeared on an MR study obtained 7 days after initiation of medical treatment with vigabatrin (Fig 2).
Symptoms and signs related to lesions of the corpus callosum, such as interhemispheric disconnection syndrome, were not present. The Table summarizes the clinical and MR imaging findings in the six patients.
Summary of clinical and MR imaging findings
Discussion
An MR imaging finding of a discrete focal nonhemorrhagic lesion within the central portion of the splenium of the corpus callosum without any accompanying lesions is unusual and is rarely encountered in any clinical setting, including epilepsy. Even though it is rare and its pathogenesis not understood, it is important to be aware of the fact that this reversible focal lesion may occur in patients with epilepsy, because it may help avoid unnecessary invasive diagnostic and therapeutic intervention.
Various pathologic conditions, such as multiple sclerosis, trauma, neoplasm, infarct, leukodystrophies (especially adrenoleukodystrophy), AIDS dementia complex, and Marchiafava-Bignami disease, may involve the corpus callosum (2–5). The MR imaging findings of these entities have been well described and appear to be different from those found in the epileptic patients in our study. In patients with early cognitive impairment due to HIV infection, abnormalities in the region of the splenium of the corpus callosum and in the crura of the fornix may be seen on MR images (3). Marchiafava-Bignami disease may cause lesions in the splenium of the corpus callosum, which are reversed when these patients receive vitamin B supplement (5). In our patients, these were excluded by both the clinical setting and the MR imaging findings.
Transient focal cerebral abnormalities on CT and MR studies have been reported in many cases of partial status epilepticus, and most of these reports have suggested transient focal cerebral edema as the basis of these abnormalities. These transient focal lesions associated with status epilepticus occur in the hippocampus or in a focal area either of white matter or of white and gray matter combined; they completely resolve after termination of status epilepticus (6–10). A focal lesion in the splenium of the corpus callosum associated with status epilepticus has never been documented.
The exact mechanism by which this lesion is produced is not clear, but it might be related to antiepileptic drugs (AEDs). In our study, the level of serum dilantin was elevated in two of four patients, and the focal lesion in the corpus callosum disappeared after withdrawal of dilantin and/or vigabatrin on the follow-up MR images in two patients. These facts suggest that the focal lesion in the corpus callosum was caused by administration of AEDs, presumably in response to either higher doses or drug sensitivity itself.
It has been reported that dilantin and vigabatrin may cause a lesion in neural tissue. A presumptive direct effect of dilantin on various portions of the nervous system has been suggested. Dilantin induces organic cerebellar damage and a distal axonopathy with secondary demyelination in the sural nerve (11, 12). In addition, dilantin may interfere with the intestinal absorption of dietary folate, causing folate deficiency, especially if other AEDs are administered concomitantly. The neurologic sequelae of folate deficiency are still controversial, but some authors have linked folate deficiency to encephalopathy, cerebellar atrophy, myelopathy, and peripheral neuropathy (13).
Vigabatrin is a new drug specifically designed to increase the brain γ-aminobutyric acid (GABA) level through a selective, irreversible inhibition of GABA transaminase. Histopathologic examination shows an intramyelinic edema (microvacuoles) in the myelinated tracts, such as the visual pathways, the hypothalamus, the fornix column, and in the white matter of the cerebellum in mice, rats, and dogs after long-term administration of high doses of vigabatrin. The intramyelinic edema disappears within a few weeks after drug withdrawal and leaves no residual effects (14–16). Weiss et al (17) reported that high-signal-intensity lesions were seen in the columns of the fornix, the hypothalamus, and the thalamus on T2-weighted MR images obtained 15 weeks after administration of vigabatrin (300 mg/kg per day) and disappeared 12 or fewer weeks after discontinuation of vigabatrin in the dog model, which was consistent with the regional patterns of vigabatrin-induced intramyelinic edema as demonstrated by histopathology. Despite extensive toxicity studies, vigabatrin-induced intramyelinic edema has yet to be observed in humans (14, 18, 19). In our study, the lesion was observed only within the splenium of the corpus callosum, but not in the other areas. A small area within the lesion showing isointensity on both T1- and T2-weighted images was considered to be a spared part of the white matter (Fig 1B and C).
Chason et al (1) reported a focal well-defined focus of T2 hyperintensity in the midportion of the splenium of the corpus callosum in association with seizure activity in epileptic patients, similar to that seen in our study. These authors postulated that it might be a transient interictal focal white matter edema in association with a transhemispheric connection of seizure activity, because five of the seven patients had seizures with secondary generalization, and the corpus callosum is known to be a major pathway of propagation of seizure activity. Our study does not support their assumption. First, five of our six patients had no secondary generalization of seizure activity. Second, even though the corpus callosum may play a role as a major pathway of propagation of seizure activity to the contralateral side, there is evidence that seizure discharge of temporal lobe origin does not propagate to the other hemisphere through the corpus callosum in human or animal models (20). Third, considering the frequency of secondary generalization of seizure activity, the relative prevalence of focal lesions in the splenium of the corpus callosum as seen in our cases seems to be too low. As described earlier, we believe that the focal lesion in the corpus callosum is a transient, reversible demyelination, presumably resulting from toxicity or drug sensitivity of AEDs or from some other, as yet undiscovered, causes.
Conclusion
A discrete focal nonhemorrhagic lesion in the splenium of the corpus callosum may be seen on MR images of patients with epilepsy. This lesion might be due to reversible demyelination related to AEDs toxicity.
Footnotes
↵1 Supported by a grant from the Medical Technology Development Research Fund, Ministry of Health and Welfare (HMP-96-M-2-1039).
↵2 Address reprint requests to Kee-Hyun Chang, MD, Department of Radiology, Seoul National University College of Medicine, 28 Yongon-dong, Chongno-gu, Seoul 110–744, Korea.
References
- Received May 26, 1998.
- Copyright © American Society of Neuroradiology