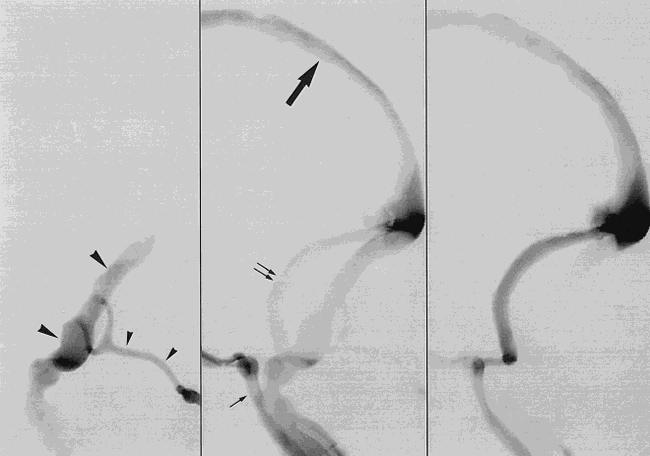Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to create and test an in vitro model of intracranial arteriovenous fistulas (AVFs) that simulates the geometry of human vasculature and allows realistic testing of devices used in endovascular therapy.
METHODS: The models were derived from corrosion casts of the main cervicocranial arteries and veins obtained from two nonfixed human specimens. Wax copies of the casts were produced and combined to create complex models simulating various types of intracranial AVFs. Wax assemblies were embedded with liquid silicone solidified into transparent blocks containing, after wax evacuation, hollow reproductions of the original vascular trees. The models were connected to a pulsatile pump and their compatibility with various imaging techniques and endovascular treatment materials was evaluated.
RESULTS: The models were compatible with digital subtraction angiography, CT, MR imaging, and transcranial Doppler sonography. They provided a realistic endovascular environment for the simulation of interventional neuroradiologic procedures.
CONCLUSION: Anatomically accurate and reproducible in vitro models of intracranial AVFs provide a valuable method for evaluating new endovascular treatment materials and for teaching purposes.
The development and preclinical evaluation of new endovascular treatment methods have created the need for reproducible, readily available experimental models of intracranial vascular disease. Although in vivo models remain an ideal environment for such studies, the use of living animals presents many disadvantages, such as high cost, poor reproducibility, and difficult ethical considerations. Major problems with animal models are related to the technical skills required for their preparation, the variable success rate achieved with them, and the inability to reproduce exactly the anatomic conditions of vascular disease. In addition, procedures simulated on animal models often necessitate an elaborate and expensive technical platform (dedicated angiographic device, general anesthesia facility). While a few animal models of intracranial vascular malformations are available (1–3), they do not provide a realistic hemodynamic and morphologic environment, since animal models (usually rats or swine) do not duplicate the conditions encountered in human clinical practice.
Simple in vitro models of various shapes (straight or curved segments, bifurcations) and materials (glass, plexiglass, silicone tubing) have been used for a long time, but their utility is confined mainly to fundamental hemodynamic investigations (4). By applying complex molding procedures to anatomic vascular casts, in vitro models accurately reproducing the human vascular system can be obtained (5–8). Such in vitro vascular models are easy to handle, respect human morphologic characteristics, and are fairly reproducible (8). They have been shown to provide realistic experimental settings for hemodynamic studies (9–11), and may also represent an interesting alternative to animal models for the evaluation of endovascular treatment methods, with the exception of biocompatibility studies (12).
We describe the development of in vitro models that reproduce some of the anatomic features associated with different types of intracranial arteriovenous fistulas (AVFs). The compatibility of these models with current imaging techniques and their capacity to provide a realistic endovascular environment for the simulation of interventional procedures are evaluated.
Methods
Model Preparation
The models were derived from arterial and venous corrosion casts obtained from two nonfixed human specimens. A mixture of methylmethacrylate (Beracryl, Troller, Switzerland) and barium sulfate powder (HD 200 plus, Lafayette Pharmacal, Lafayette, IN) was injected under fluoroscopic control into the descending thoracic aorta of a 72-year-old female specimen for the arterial cast, and in both internal jugular veins of a 70-year-old male specimen for the venous cast. Once the injected material solidified, the specimens were immersed in a 15% solution of potassium hydroxide and maintained at 40°C until the surrounding soft and bony tissues were completely eliminated. We then streamlined the corrosion casts by removing the small branches, leaving cleaned casts consisting of the main cervicocranial arteries or veins. From these casts, two separate vascular trees were individualized for the preparation of the models: a cast of the right internal carotid artery (ICA) with its main branches and a cast of the principal dural venous sinuses, also including the right superior ophthalmic vein and the right superficial middle cerebral vein. Multiple wax copies of these vascular casts were then produced by applying a molding technique adapted from dentistry prosthetic devices (Elastrat, Geneva, Switzerland). Using various combinations of these arterial and venous wax copies, we created complex models covering the three etiologic groups of intracranial AVF: 1) congenital fistulas, or vein of Galen aneurysmal malformations; 2) acquired fistulas, or dural arteriovenous fistulas (DAVFs) of the lateral and cavernous sinuses; and 3) traumatic fistulas, or carotid cavernous fistulas (CCFs). Separate walls for the cavernous segment of the ICA and the cavernous sinus itself were obtained in some models by coating the ICA wax copies with a thin layer of silicone (Elastosil RT601, Wacker Chemie Ag, Germany) before assembling the arterial and venous components. The various wax elements constituting a complex model were held together by hot wax deposited between the parts that were to be connected, such as the distal extremity of an arterial feeder and the vein of Galen aneurysmal pouch. To simulate a parenchymal phase, a delay in the circulation time between the arterial and venous phases was created by the interposition of silicone tubing (1 mm in diameter, 30 cm in length) between the distal extremity of some of the ICA branches and the anterior end of the superior sagittal sinus. The complex wax models were finally secured in plexiglass boxes and embedded with liquid silicone, which rapidly solidified into transparent blocks (Elastosil RT601). Once solidified, the blocks were heated (to about 80°C) to melt and evacuate the wax through previously drilled holes.
The models were then connected to a pump (Drapier Type, Collin, France), which delivered a pulsatile flow, while the type of circulating fluid was chosen according to the investigation undertaken: simple water was generally used for imaging studies such as digital subtraction angiography (DSA), while a complex liquid kept at 37°C and reproducing the rheologic properties of human blood was used either for sonographic studies or when the behavior of new endovascular therapeutic devices, such as detachable coils, was to be evaluated. This non-Newtonian fluid consisted of an aqueous suspension of polystyrene prepared according to Fukada et al (13) in a mixture of 12% polystyrene microparticles (diameter, 10 μm) (Across, Belgium), 5% dextran, and 10 mMol calcium chloride. Previous evaluations proved that this fluid could be used with DSA, CT, MR imaging, transcranial Doppler sonography, and endovascular materials.
Compatibility with Imaging Equipment
The imaging compatibility of the models was evaluated with DSA (Integris BN3000, Philips, the Netherlands), CT (PQ 5000, Picker, Highland Heights, OH), MR imaging (Edge, Picker), and transcranial Doppler sonography (Acuson XP128, Acuson, Mountain View, CA).
Compatibility with Endovascular Treatment Techniques
The capacity of the models to allow realistic simulation of conditions encountered during endovascular procedures was tested by applying a variety of endovascular medical devices, either in current clinical use (microcatheters, guidewires, balloons) or in a preclinical evaluation phase (detachable coils).
Model Evaluation by Interventional Radiologists
Four different characteristics of the model were evaluated by nine investigators during the preclinical trial of a new endovascular treatment device (Detach-18, Cook Europe, Denmark). The investigators were interventional neuroradiologists from nine different European university medical centers. Investigators working in our institution were not included in the evaluation protocol. The investigators were asked to anonymously complete a questionnaire evaluating the ability of the models to reproduce complex vascular structures by examining the silicone blocks (to assess morphologic accuracy) and by performing angiography in the models (to assess angiographic accuracy). Two other characteristics concerning the ability of the models to provide a realistic endovascular environment as compared with in vivo situations (catheter navigation and microcoil placement) were also evaluated during embolization procedures.
Results
Vascular Anatomy Simulation
The in vitro models were created by using casts reproducing the vascular lumen of human specimens. Potential alteration of the vascular morphology due to the anatomic preparation itself (ie, excessive injection pressure of the casting material) was avoided by continuous fluoroscopic control of the procedure, which could thus be interrupted as soon as the relevant vessels were filled. The molding technique applied thereafter allowed for the production of an unlimited number of wax copies reproducing with great accuracy the three-dimensional branching pattern as well as the minute surface irregularities of the initial vascular cast.
Vascular Disease Simulation
In vitro models reproducing some of the anatomic features associated with complex vascular disorders, such as AVFs, were obtained by combining arterial and venous wax copies. DAVF simulations were created by establishing multiple direct connections between the arterial and venous components of the models (Figs 1 and 2). In some models, the venous wax copies were modified to reproduce some dural sinus alterations associated with typical patterns of DAVF venous drainage (types I and II). These alterations included luminal stenosis or occlusion. Simulation of a CCF was more complex, requiring an initial preparation of a thin wall for the intracavernous portion of the ICA (segments C3 to C5). Single holes of various sizes, shapes, and locations were made in the walls of the different models to reproduce the fistulous connection between the ICA and the cavernous sinus. To prepare models simulating vein of Galen aneurysmal malformations, spherical pouches of different diameters (2 and 3 cm) were added to the venous component and connected to multiple arterial branches of the anterior and posterior cerebral arteries (Fig 2A–C). The interposition of silicone tubing between some arterial branches and the superior sagittal sinus allowed the simulation in all models of a normal venous phase secondary to the early venous filling resulting from the presence of an arteriovenous shunt.
DSA, lateral projection, of in vitro model simulating the anatomic features of a DAVF of the right transverse sinus. The arterial phase (left) reveals early filling of the right transverse sinus (large arrowheads) by fistulous arterial branches (small arrowheads). The venous phase (center) shows opacification of the superior sagittal sinus (large arrow), the inferior petrosal sinuses (small arrow), and the left transverse sinus (double arrow). In late venous phase (right), the contrast material is washed out of the right transverse sinus by nonopacified fluid coming from the arteriovenous shunt
DSA, lateral projections, of in vitro model that combines some of the features associated with both a CCF and a vein of Galen aneurysmal malformation.
A, The arterial phase reveals early filling of the right cavernous sinus due to the simulated CCF, and partial opacification of the reproduction of the vein of Galen aneurysmal malformation (arrowhead).
B, The late arterial phase shows complete opacification of the reproduction of the vein of Galen aneurysmal malformation by multiple arterial feeders coming from the anterior (large arrow) and posterior (small arrow) cerebral arteries.
C, Venous phase.
D, Road-map image shows coil placement. Several detachable coils have been placed in the simulation of the vein of Galen aneurysmal malformation through a transvenous approach. The microcatheter can be seen in the straight and right transverse sinuses, with its distal aspect lying in the vein of Galen aneurysmal pouch.
Use of the Models for Medical Imaging Evaluation
The models were compatible with X-ray–based imaging methods (DSA and CT), MR imaging, and sonography (TCD). Since the models are made of transparent silicone, procedures performed on them may also be monitored optically, such as with photography or videorecording. On DSA studies, sequential opacification of the arterial and venous components caused by the interposition of the silicone tubing, acting like a “capillary phase,” appeared very realistic.
Use of the Models for Endovascular Medical Device Evaluation
Endovascular materials either currently in use (different types of catheters and microcatheters, guidewires, and occlusion balloons) or in a preclinical evaluation phase (microcoils) (Fig 2D) were used under conditions closely approaching in vivo situations. In particular, endovascular manipulations, such as catheter and guidewire navigation, were subjectively regarded as very realistic, despite the lack of elasticity of the solid models. The evaluations by the nine interventional neuroradiologists revealed overall excellent acceptance of the model for its morphologic qualities. The models were reported to provide a realistic in vitro environment that matched the conditions encountered in comparable clinical situations, and they were also considered an ideal environment for medical teaching purposes.
Discussion
In vitro models that reproduce the morphologic complexity of the human cerebral vasculature can be created by using arterial and venous corrosion casts obtained from human specimens. Fluoroscopic control of the anatomic procedure prevents overdistension and distortion of the injected vessels. In our study, the choice of methylmethacrylate as the corrosion cast material allowed accurate reproduction of the irregularities of the internal vascular surface (15). The in vitro models were derived from vascular corrosion casts by applying a molding procedure, as previously proposed by Liepsch and Zimmer (5) and by Kerber et al (6). The capacity of our methodology to provide an unlimited number of wax copies from an initial cast allows for the production of highly reproducible models, as, for example, required for comparative testing. Previous studies have shown that the variation in caliber induced by the different procedures used in the preparation of the models (from the corrosion cast to the final transparent blocks) was negligible (less than 4% of the initial caliber) (8). This reproducibility also allows one to perform selective modifications on the wax copies, such as changing the size, shape, or position of the vessels, and to study the influence of these changes on the imaging and endovascular properties of the models.
By combining reproducible and anatomically accurate arterial and venous wax copies, complex in vitro models simulating vascular disorders, such as intracranial AVFs, were created, providing a valuable alternative to in vivo models. A wide range of neurovascular diseases may be reproduced in in vitro conditions by combining an accurate anatomic environment with a realistic simulation of the pathologic state. The in vitro methodology also provides precise control over the intrinsic characteristics of the simulated diseases, such as lesion size or caliber and number of feeding arteries, as well as control over such physiological parameters as fluid pressure and flow velocity. The in vitro models, however, do not realistically reproduce the hemodynamic conditions associated with complex arteriovenous connections. Present limitations of in vitro models include the inability to simulate diseases involving small vessels (less than 2 mm in diameter) and to assess host biological response to new medical devices (biocompatibility testing).
Because the in vitro models exhibited realistic characteristics with currently available imaging (DSA, CT, MR imaging) and flow measurement (transcranial Doppler sonography) techniques, they may be considered valuable tools for the development and evaluation of new imaging methods, as well as for the acquisition of hemodynamic studies based on sonographic and MR angiographic techniques.
The excellent endovascular properties shown by the in vitro models when tested with materials currently in use (catheters, guidewires, balloons) confirm that they provide a realistic environment for the development and evaluation of new endovascular medical devices. Although models with silicone blocks are less realistic than those with thin soft silicone walls when viscoelastic properties are considered, the lack of elasticity of the arterial walls was not thought to affect significantly the realism of the endovascular manipulations. Catheter navigation in the ICA was even considered smoother and more realistic than in corresponding soft models, probably because of a decrease in friction due to the greater stability of the wall relative to the soft thin-walled models. Moreover, silicone blocks are probably more appropriate than soft models for simulating the characteristics of rigid dural sinus walls. In the CCF models, the intracavernous segment of the ICA had thin silicone walls with viscoelastic properties that closely reproduced those of real human carotid arteries (16). Endovascular manipulations inside the cavernous sinus, such as balloon or coil delivery, were thus performed in an environment that combined realistic arterial and dural wall characteristics.
The results of the evaluation performed by the experienced interventional neuroradiologists showed an overall excellent acceptance of the model as anatomically accurate and as able to provide realistic conditions for experimental studies, such as device testing. These anatomically accurate in vitro models also appear to be excellent educational tools for endovascular interventional training. They enable the physician to study the correct relationships of three-dimensional vascular structures and to gain hands-on experience in various endovascular techniques, including the interventional approach of rare and difficult to treat neurovascular diseases, such as vein of Galen aneurysmal malformations.
Conclusion
Anatomically accurate and highly reproducible in vitro models of human intracranial AVFs provide a valuable method for the development and initial evaluation of new treatment materials, imaging methods, hemodynamic investigations, and teaching techniques. This type of in vitro model has the potential to replace animal models for most medical training and device testing (excluding biocompatibility evaluation) related to neurovascular disease involving medium to large vessels, such as intracranial AVFs.
Acknowledgments
We thank L. Auer, J-M. Meyer, and W. Weber for their valuable scientific contributions to the present work.
Footnotes
↵1 Supported by a grant from the Swiss National Science Foundation (Project #32–42529.94).
↵2 Presented at the annual meeting of the American Society of Neuroradiology, Toronto, Canada, May 1997.
↵3 Recipient of the annual award of the Swiss Society of Medical Radiology (1997) and a special mention of the French Society of Radiology (1997).
↵6 Address reprint requests to Philippe Gailloud, MD, Neuroradiology Division, Johns Hopkins Hospital, 600 N Wolfe St, Baltimore, MD 21287.
References
- Received February 9, 1998.
- Copyright © American Society of Neuroradiology