Abstract
BACKGROUND AND PURPOSE: Previous reports have suggested that thyroglossal duct cysts (TDCs) appear on sonograms as well-defined cystic masses with thin walls and posterior enhancement. In our experience, however, TDCs have a variable sonographic appearance. We report our findings in 40 patients with TDCs and document the variability of sonographic patterns.
METHODS: All patients in whom the diagnosis of TDC was made clinically (by at least two head and neck surgeons) and sonography detected a cystic mass related to the hyoid bone were included in this study. Sonograms of 40 patients with TDCs were reviewed. The features evaluated were the location, internal echogenicity, posterior enhancement, presence of septa, solid component, and fistulous tract. The echo pattern was not compared with the biopsy results.
RESULTS: Four patterns of TDCs were identified: anechoic (28%), homogeneously hypoechoic with internal debris (18%), pseudosolid (28%), and heterogeneous (28%). The majority showed posterior enhancement (88%), were midline (63%), and infrahyoid in location (83%). Only half of all TDCs showed a typical thin wall.
CONCLUSION: On sonograms, TDCs in adults are not simple cysts, as previously suggested, but have a complex pattern ranging from a typical anechoic to a pseudosolid appearance.
Thyroglossal duct cysts (TDCs) are diagnosed clinically. The role of imaging is to confirm the clinical diagnosis and identify the presence of the thyroid gland. It can also provide preoperative information regarding the presence or absence of a solid component within the cyst.
Although the role of CT and MR imaging is well documented (1, 2), high-resolution sonography remains the ideal initial investigation because it is easily available, inexpensive, and provides the surgeon with necessary preoperative information. In adults, TDCs have previously been described as well-defined, smoothly outlined, and uniformly anechoic with posterior enhancement (3). However, we have found that TDCs in adults have a variable sonographic appearance. This article documents that range of characteristics.
Methods
The sonograms of 40 patients with TDCs were reviewed retrospectively. All the scans were done by the same sonologist; they were reviewed by two radiologists with 8 and 3 years experience in head and neck sonography, respectively, and the results were obtained by consensus. Because TDCs are diagnosed clinically, sonography completes the physical examination. Patients in this study, as in previous studies (4), were included on the basis of clinical and sonographic findings. All patients had been examined by at least two head and neck surgeons who independently made the diagnosis of TDC, and all had sonographic findings that suggested a TDC in the vicinity of the hyoid bone. At the time of their sonograms, none of the patients had any clinical evidence of infection or a history of thyroid carcinoma or thyroid surgery. The study group included 22 women and 18 men; ages ranged from 19 to 87 years.
The patients were scanned supine with their necks hyperextended on a pillow. All scans were performed with a 7.5- or 10-MHz transducer, and images were obtained in the transverse and longitudinal planes.
The sonograms were evaluated for the following features: site of the mass, size, walls, margins, loculation, internal echogenicity, posterior enhancement, internal septa, solid component, presence or absence of the thyroid, and any fistulous communication.
The site was characterized in relationship to the hyoid bone and to the midline. Internal echogenicity was characterized as anechoic (strictly no internal echoes), homogeneously hypoechoic (lower amplitude than surrounding tissues, these were predominantly cystic with low-amplitude internal echoes), homogeneously hyperechoic or pseudosolid (more echogenic than adjacent tissues), and heterogeneous. Wall thickness was defined as imperceptible, thin (1–2 mm), or thick (2 mm or greater). The well-defined margin of the cyst was considered to be the outer wall. If the inner wall was seen only in a small portion of the cyst, the wall thickness was defined as imperceptible.
After sonography, 11 patients also had fine-needle aspiration cytology (FNAC) of the cystic nodule, which confirmed a TDC. In two patients, the diagnosis was confirmed by surgery. No evidence of malignancy was detected in any of these cases. The remaining patients refused surgery and FNAC, as the cysts were small, long-standing, and did not cause them any problems. They continue to be followed up in the surgical clinic, and thus far follow-up sonographic examination has shown no change in appearance. They are well and otherwise asymptomatic, and the diagnosis remains TDC.
Results
Site
Of the 40 lesions, 25 (63%) were located in the midline and 15 (38%) were off midline (11 were located to the left of the midline and four to the right). Thirty-three (83%) were infrahyoid in location, five at the level of the hyoid (13%), and two (5%) were suprahyoid.
Size
TDCs ranged in size from 8 to 33 mm.
Walls, Margins, and Loculation
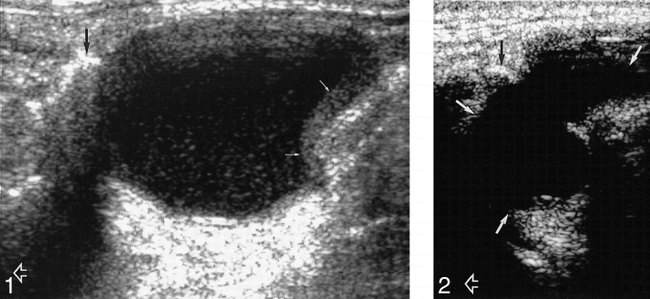
Of the 40 lesions, 20 (50%) had thin walls, 18 (45%) were thick-walled (Fig 1), and in two lesions (5%) the walls were imperceptible. All 40 lesions had well-defined margins. Thirty-five (88%) of the cysts were unilocular and five (12%) were multiloculated (Fig 2).
Longitudinal sonogram of a predominantly anechoic TDC with internal debris and thick walls (solid white arrows) below the level of the hyoid bone (black arrow). Open arrow indicates the head end of the patient.
fig 2. Longitudinal sonogram of an anechoic, multiloculated TDC (solid white arrows) with extension posterior to the hyoid bone (black arrow). Open arrow indicates the head end of the patient.
Internal Architecture
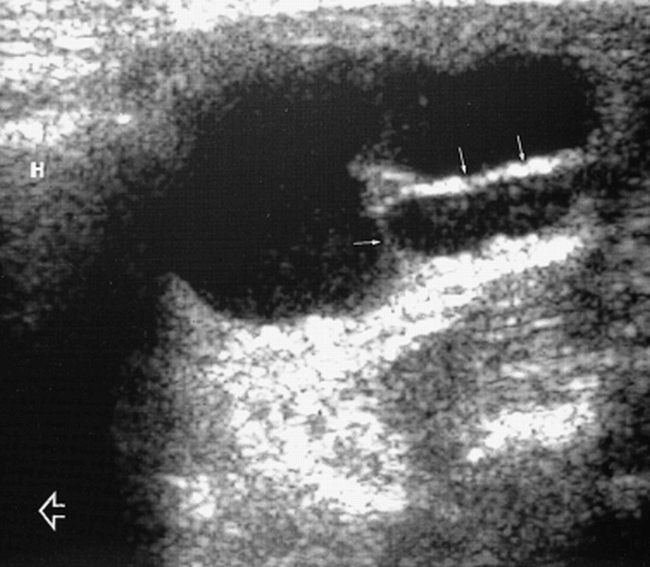
Four patterns of TDCs were identified: 1) Eleven lesions (28%) were truly anechoic (Fig 2) with posterior enhancement. 2) Seven lesions (18%) were predominantly homogeneously hypoechoic but contained low-amplitude freely floating debris (Fig 1) and had posterior enhancement. 3) Eleven lesions (28%) were hyperechoic and had a pseudosolid appearance (Fig 3). Only six of these showed posterior enhancement; however, after application of transducer pressure on the cyst, the entire contents shifted in all cases, suggesting their true cystic nature. 4) Eleven lesions (28%) showed heterogeneous internal echoes with coarse internal debris and septa (Figs 4 and 5) and posterior enhancement. These were the 11 lesions in which FNAC was performed, and in all cases, whitish material was aspirated from the cyst.
Transverse sonogram of a uniformly echogenic, pseudosolid appearance of a TDC (large arrows). Note, however, the posterior enhancement (small arrows), suggesting its cystic nature.
fig 4. Longitudinal sonogram of a TDC shows a mixed echo pattern with internal debris (arrowhead) and septa (solid arrows). Note its relationship to the hyoid bone (H) and the intense posterior enhancement. Open arrow indicates the head end of the patient.
Longitudinal sonogram of a TDC shows thick septa (solid arrows). Note its relationship to the hyoid bone (H) and the intense posterior enhancement. Open arrow indicates the head end of the patient
At sonography, none of the TDCs in this series had a solid component.
Fistulous Tract
A fistulous tract was identified by sonography in only one patient.
Thyroid Status
In seven patients (18%) the thyroid showed changes suggestive of nodular hyperplasia, whereas the thyroid was normal in the remaining 33 patients (83%).
Discussion
The most common congenital anomaly related to the thyroglossal duct is the TDC. It is thought to represent segments of the duct that fail to regress and consequently differentiate into epithelial-lined cysts. TDCs develop anywhere along the course of the duct remnant, from the base of the tongue to the suprasternal region (5). Cysts located near the foramen caecum are lined by stratified squamous epithelium, whereas cysts located near the thyroid gland are lined by cells similar to thyroidal acinar epithelium. Functional thyroid tissue within the TDC has been described (6) and more than half contain normal thyroid tissue in their walls (7).
TDCs in this study were most commonly midline, but 38% were slightly off midline. In accordance with the literature, we found that the majority of off-midline cysts are characteristically located adjacent to the outer surface of the thyroid cartilage, deep to the strap muscles. TDCs are located in the region of the hyoid bone. About 20% to 25% are suprahyoid, 15% to 50% occurring at the level of the hyoid bone, where they may be anterior or posterior to the hyoid bone, and 25% to 65% occurring in the infrahyoid part of the neck (8). Contrary to previous reports, we found that TDCs in adults are more likely to be infrahyoid in location (82%), decreasing in frequency with ascension up the neck, with only 5% in a suprahyoid location.
The typical sonographic description of a TDC has been that of an anechoic, well-circumscribed cyst with increased through-transmission (3, 9–11). However, earlier studies in children have shown that most are not simple cysts but instead are either homogeneous or heterogeneous complex hypoechoic lesions (5). In the present study, only 11 lesions were truly anechoic, another seven were predominantly anechoic but contained internal debris, 11 had a complex heterogeneous echo pattern, and 11 had a uniformly homogeneous pseudosolid appearance.
The pseudosolid appearance of cystic lesions has been described previously for other congenital cystic lesions in the neck, such as branchial cleft cysts (12) and dermoid cysts, but not for TDCs. In dermoids and branchial cleft cysts, the echogenic appearance is due to the presence of cellular material, cholesterol crystals, and keratin within the cyst. In TDCs, this appearance may be due to the proteinaceous content of the fluid, thought to be secreted by the epithelial lining of the cyst (5). The uniform echogenicity may lead to an erroneous assumption that the lesion is solid, especially when posterior enhancement is absent, as seen in almost half these cases. However, when pressure is applied on the cyst with the transducer, the entire contents shift, suggesting its cystic nature.
Eleven patients in this study had TDCs that showed a complex echo pattern due to coarse internal debris and septa. Aspiration of such complex cystic lesions yielded whitish material rather than altered blood. This corresponds to the previous study and indicates that the coarse internal echoes seen in TDCs are not due to altered blood but that the echo pattern is due to the proteinaceous content of the cyst secreted by the cyst lining (5).
Intense posterior enhancement is a characteristic feature of an uncomplicated cyst. In this study posterior enhancement was present in 88% of the cases and easily identified in lesions that were anechoic or had mixed echogenicity. However, in lesions that had a pseudosolid appearance, the posterior enhancement was often subtle and was the key to identifying the cystic rather than solid nature of the nodule. Often it is difficult to identify the posterior enhancement, particularly if the lesions are in proximity to the airway (5). In this study, posterior enhancement was not seen in five (13%) of the 40 cases, all of which had a pseudosolid appearance.
All the TDCs in this study were well defined. In 50% of the lesions, the walls were thin; 45% were thick-walled; and in the remaining 5%, the walls were imperceptible. Previously, it was believed that thick walls were predominantly due to infection or hemorrhage. However, this study and another (6) cannot confirm hemorrhage as a likely cause. It is more likely that the thick walls are due to inflammation and cellular debris. The majority of cysts in this series were unilocular (88%), and only a small portion were multilocular.
At sonography, none of the TDCs in this study had a solid component. The presence of a solid component should alert the sonologist to the possibility of a TDC carcinoma, as malignant degeneration of the epithelial lining of a TDC (usually into a papillary carcinoma) has been reported as a rare complication (13, 14). Although a sonographically guided FNAC performed on any such solid component would identify a malignant lesion, it may not be necessary in patients undergoing surgery, as the cyst and the solid component would be resected. The treatment recommended is a near total or total thyroidectomy (following a Sistrunk procedure) and sampling of adjacent nodes because of the possibility of intrathyroidal foci of cancer (15).
Although sonography does not reveal a tract in all cases, this is not critical because, irrespective of the site or the size and appearance of a TDC, a Sistrunk procedure is the recommended procedure of choice (16–19). This entails resection of the cyst and any remaining tract, and excision of the middle third of the hyoid bone. Incomplete resection invariably results in recurrence. With the use of the Sistrunk procedure, recurrence rates have fallen from 50% to less than 4% (16).
The differential diagnosis of TDC in adults includes dermoid cyst, branchial cleft cyst, lymphadenopathy, and a cystic nodule arising from the thyroid gland. Lymph nodes are often multiple, hypoechoic, and show the presence of an echogenic hilus. In cases in which the hilus is absent, the distribution, appearance, and presence of other nodes identify the nature of the nodule. Lymphomatous nodes may also show posterior enhancement (20); however, the intranodal morphology, distribution, and presence of other nodes provide a clue to the nature of the node. Midline cystic thyroid lesions are also readily identified by sonography; they are often accompanied by other lesions in the thyroid as a part of nodular hyperplasia. Branchial cleft cysts may be difficult to distinguish from off-midline TDCs; however, the characteristic location and FNAC are helpful in differentiating the two. Although previous reports suggest that FNAC must be performed only in patients who agree to subsequent surgery (because FNAC without subsequent surgery may lead to the formation of fistulas [3]), this has not been our experience. Midline dermoids are also located around the hyoid bone and have an echogenic, pseudosolid appearance similar to the TDCs. Preoperative differentiation between the two is not always necessary, as this can be done at surgery. A previous report also suggests that this differentiation is not essential, since both conditions should be treated by a Sistrunk procedure to avoid incomplete excision (19).
Conclusion
The sonographic appearance of TDCs in adults is variable; to make a correct preoperative assessment, the sonologist must be familiar with these characteristics. TDCs in adults do not seem to be simple cysts, as previously suggested, but instead they have a complex cystic pattern ranging from an anechoic to a pseudosolid appearance. Sonography can provide information that the surgeon requires preoperatively without the need for other time-consuming and expensive radiologic procedures, such as CT and MR imaging.
Footnotes
↵1 Address reprint requests to Dr Anil T. Ahuja, Department of Diagnostic Radiology and Organ Imaging, Prince of Wales Hospital, Shatin N.T., Hong Kong.
References
- Received April 7, 1998.
- Copyright © American Society of Neuroradiology