Abstract
BACKGROUND AND PURPOSE: HIV enters the CNS early in the course of infection and produces neuropsychiatric impairment throughout the course of illness, which preferentially affects the subcortical white matter. The development of a neuroimaging marker of HIV may allow for the earliest detection of cognitive impairment. The purpose of this study was to determine whether MR diffusion tensor imaging can detect white matter abnormalities in patients who have tested positive for HIV.
METHODS: Ten patients with HIV (eight men and two women; mean age, 42 years) underwent MR imaging of the brain with MR diffusion tensor imaging, which included routine fluid-attenuated inversion recovery and fast spin-echo T2-weighted imaging. Diffusion constants and anisotropy indices were calculated from diffusion tensor maps. Peripheral viral load, Centers for Disease Control staging, and cluster of differentiation 4 levels were determined.
RESULTS: All patients had normal results of MR imaging of the brain, except for mild atrophy. Four of 10 patients had undetectable viral loads. These patients were receiving highly active antiretroviral therapy. The diffusion constant and anisotropy were normal. Four of 10 patients had viral loads between 10,000 and 200,000. Diffusion anisotropy in the splenium and genu was significantly decreased (P < .02). The diffusion constant of the subcortical white matter was elevated in the frontal and parietooccipital lobes (11%). Two of 10 patients had viral loads >400,000. Anisotropy of the splenium was half normal (P < .0004) and of the genu was decreased 25% (P < .002). The average diffusion constant was diffusely elevated in the subcortical white matter.
CONCLUSION: Calculating the diffusion constant and anisotropy in the subcortical white matter and corpus callosum in patients with HIV detected abnormalities despite normal-appearing white matter on MR images and nonfocal neurologic examinations. Patients with the highest diffusion constant elevations and largest anisotropy decreases had the most advanced HIV disease. Patients with the lowest viral load levels, who had normal anisotropy and diffusion constants, were receiving highly active antiretroviral therapy.
HIV is neurotrophic, and most patients who have tested positive for HIV develop neuropsychiatric impairment during the course of illness, which ranges from HIV-associated mild cognitive motor disorder to HIV-associated dementia (1–5). What triggers the transition from an asymptomatic patient to one with cognitive impairment is unknown, but the development of a neuroimaging marker of HIV disease in the CNS may allow for the earliest possible detection of cognitive impairment. This could be used, in theory, to measure response to antiretroviral therapy.
Recent proton MR spectroscopic studies have shown some promise as potential markers of HIV disease in the CNS. These techniques have shown metabolic abnormalities in patients who have tested positive for HIV that seem to correlate with early cognitive impairment (6). However, proton MR spectroscopy is not in routine clinical use. This technique is limited by the relatively long imaging time for each voxel sampled (minimum of 3–5 min), the need for skilled, accurate voxel placement or a dedicated spectroscopist, and patient compliance (no movement). Therefore, we investigated MR diffusion imaging, an emerging MR technique that is in routine clinical use. We hypothesized that diffusion tensor imaging can detect abnormalities in the corpus callosum and subcortical white matter of patients with HIV, which may correlate with either the clinical stage of disease or viral load level.
Methods
During an 8-month period, 19 patients with HIV (13 men and six women; mean age, 44 years) who had undergone MR diffusion tensor imaging were prospectively identified. Nine of these patients were excluded for the following reasons: six had periventricular white matter disease suggestive of progressive multifocal leucoencephalopathy (PML) or HIV encephalopathy, two exhibited excessive motion during the study, and one had claustrophobia and failed to complete the examination. The remaining 10 patients (eight men and two women; mean age, 42 years) underwent nonfocal neurologic examinations and had normal results of MR imaging of the brain, with the exception of age-inappropriate atrophy. The images were interpreted by a neuroradiologist without any clinical knowledge of the patients other than HIV-positive status. In addition to routine clinical MR pulse sequences, these patients underwent MR imaging that included MR diffusion tensor imaging. Of these 10 patients, four had presented with headache, four with changes in mental status, one with a questionable history of seizure that was not witnessed by the patient's clinician, and one with vertigo. Four of the 10 patients were not receiving protease inhibitors. The remaining six patients were receiving various combinations of highly active antiretroviral therapy (HAART). Cluster of differentiation 4 (CD4) levels and viral loads (HIV messenger RNA) in the blood were obtained from all patients. Centers for Disease Control staging was determined from clinical information that was obtained from chart review.
All 10 patients underwent routine MR imaging on a 1.5-T magnet using a standard head coil. Routine sequences were performed, including sagittal T1-weighted (500−600/12/1 [TR/TE/excitations]), axial fast spin-echo T2-weighted (3000/91/1), axial fluid-attenuated inversion recovery (10002/172/1) with an inversion time of 2200 ms, axial T1-weighted (500/14/1), and axial diffusion-weighted echo-planar (6000/99−100/1) imaging. In addition, a diffusion tensor pulse sequence, which can measure the diffusion in any arbitrary direction, was then used. The sequence is a single-shot, multisection, spin-echo, echo-planar pulse sequence (6000/100/1), using a matrix size of 128 × 128, 5-mm interleaved data acquisition, 30 sections to cover the whole brain, and a field of view of 22 cm. Using this sequence, we collected the diffusion-weighted images in seven directions (X, Y, Z, X+Y, X+Z, Y+Z, X+Y+Z), with a maximum b value of 820 s/mm2 per gradient axis. The data acquisition time for this part of the examination is 5 to 10 minutes, depending on the number of diffusion-weighted images acquired. For most of the cases, we used a total of 22 diffusion-weighted images with differing b values.
On a workstation, an orientation-independent diffusion map (Dav = Trace/3 = [Dxx + Dyy + Dzz]/3) was calculated for each pixel from the diffusion-weighted images. Using a multivariate fitting routine, in C, we calculated six diffusion maps corresponding to the six independent elements of the diffusion tensor, D̄. From these diffusion maps, we calculated an orientationally invariant average diffusion map and an orientationally invariant anisotropy map using anisotropy index, UAsurf. This anisotropy index is calculated by comparing the Dav with the Dsurf, which is a new diffusion constant obtained from the surface of the diffusion ellipsoid (7, 8). This anisotropy index, which has high sensitivity, is defined in terms of diffusion coefficients as follows:  Diffusion anisotropy (UAsurf) is scaled between 0 to 1, where 0 corresponds to isotropic diffusion and 1 to fully anisotropic unidirectional diffusion.
Diffusion anisotropy (UAsurf) is scaled between 0 to 1, where 0 corresponds to isotropic diffusion and 1 to fully anisotropic unidirectional diffusion.
There is no standard, accepted way to measure anisotropy. Different groups have chosen to use different anisotropy measures, such as relative anisotropy index or fractional anisostropy to describe anisotropy (9–11). Recent studies have shown that different anisotropy measures have different sensitivities in describing tissue anisotropy, and the anisotropy index, (UAsurf), is the most sensitive measurement for the detection of anisotropy changes in white matter fiber tracts (7, 12, 13). Because we were interested in white matter fiber tracts, we chose to use this anisotropy index.
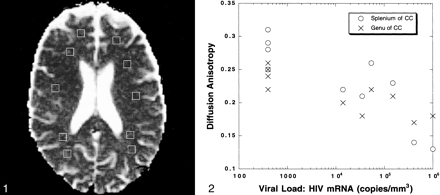
For each patient, average diffusion constants were calculated from the following regions of interest using voxel sizes of 3 to 5 mm: the frontal lobe subcortical white matter bilaterally, the parietooccipital subcortical white matter bilaterally, the centrum semiovale bilaterally, and the genu and splenium of the corpus callosum (Fig 1). These values were compared with normative data of adults, one of whom has been reported in the literature by one of the authors in this study (A.M.U.) (7). Two of the authors measured the diffusion constants and anisotropy values without any clinical knowledge regarding the patients except for the HIV-positive status. The mean values of the diffusion constant and anisotropy for the patients with lowest, intermediate, and highest viral load levels were compared using a Student's two-tailed t test, assuming unequal variance and using a P level of <.05 for significance.
Voxel placements are shown on the diffusion tensor sequence for the determination of the average diffusion constant (Dav).fig 2. Graph plots values for the anisotropy in the genu and splenium of the corpus callosum versus the measured peripheral viral load levels (HIV messenger RNA copies/mm3). An inverse trend is observed. As the viral load levels increase, the anisotropy of these structures decreases
Results
Table 1 provides a summary of the clinical data of these patients with HIV, including CD4 counts, viral load levels, and Centers for Disease Control staging. Tables 2 and 3 list the means and SD for the measurements of the diffusion constant (Dav) and anisotropy (UAsurf) that were obtained in the subcortical white matter and corpus callosum.
Clinical data
Diffusion constant (Dav) measurements (10−5 cm2/s)*
Anisotropy measurements*
All patients had normal results of MR imaging of the brain, except for mild atrophy, and all patients had nonfocal neurologic examinations. Six of 10 patients were receiving HAART.
Four patients had undetectable peripheral viral load levels (<400 copies/mm3). These patients had an average CD4 count of 489, and all were receiving HAART. Using Centers for Disease Control criteria, two had stage A1 disease and two had stage C3 disease (AIDS). In these patients, the diffusion constant (Dav) in the frontal lobe subcortical white matter was only slightly elevated (4% increase) to 0.78 × 10−5cm2/s. In the parietooccipital subcortical white matter and centrum semiovale, the Dav was normal. In these patients, there was an elevation of the Dav in the genu of the corpus callosum to 0.83 × 10−5 cm2/s, which is 11% higher than expected. However, the splenium of the corpus callosum showed a decrease in Dav to 0.76 × 10−5 cm2/s. Notably, the anisotropy within the genu and splenium was normal (Tables 2 and 3).
Four patients had detectable viral load levels between 10,000 and 200,000 HIV messenger RNA copies/mm3. These patients had an average CD4 count of 522, and only one was receiving HAART. Of these patients, one had Centers for Disease Control stage A1 disease, another had stage B2, and two had full-blown AIDS (stages C1 and C3). These patients, who had intermediate viral load levels, had elevations in the diffusion constant in the frontal subcortical white matter (0.84 × 10−5 cm2/s), which represents an increase of 12% compared with normal. In the parietooccipital subcortical white matter, a smaller 8% increase was noted (Dav of 0.81 × 10−5 cm2/s). None of these increases, however, were statistically significant compared with normal values. The Dav in the centrum semiovale was normal. In the genu of the corpus callosum, the Dav was slightly elevated to 0.80 × 10−5 cm2/s (6% increase) but the anisotropy decreased by 17% (UAsurf of .20), which was statistically significant (P < .02). The Dav within the splenium of the corpus callosum is decreased to 0.81 × 10−5 cm2/s, but the anisotropy was 0.23, which represents an 18% decrease compared with normal values, and this was statistically significant (P < .02) (Tables 2 and 3).
Two patients had significantly high viral load levels (>400,000 copies HIV messenger RNA/mm3). These patients had very low CD4 counts of 6 and 30, respectively, and one was not receiving HAART. Both patients had Centers for Disease Control stage C (AIDS). These patients had large increases in the Dav at all measured locations despite a normal-appearing brain on MR images. The frontal subcortical white matter was increased to 1.07 × 10−5 cm2/s, which is 44% above normal. In the parietooccipital subcortical white matter, the Dav was elevated to 1.13 × 10−5 cm2/s, which represents an increase of 51%. In the centrum semiovale, the Dav was elevated by 23% (0.86 × 10−5 cm2/s). In the genu of the corpus callosum, the Dav was elevated to 1.03 × 10−5 cm2/s, and, in the splenium of the corpus callosum, the Dav was 1.10 × 10−5 cm2/s, which was of statistical significance (P < .02). The anisotropy of the genu was decreased to 0.18, which is a 25% decrease from normal and is statistically significant (P < .002). The anisotropy of the splenium of the corpus callosum was decreased to 0.14, which is a 50% decrease from normal and is of a high degree of statistical significance (P < .0004) (Tables 2 and 3).
In the measurements of the diffusion constant, there were no differences detected regarding right versus left side of the brain. Thus, single means are indicated for the frontal and parietooccipital subcortical white matter, the centrum semiovale, and the genu and splenium of the corpus callosum.
Discussion
Early in the course of infection, HIV enters the CNS and produces neuropsychiatric impairment throughout the course of illness for most patients with AIDS, which ranges from HIV-associated mild cognitive motor disorder to HIV-associated dementia (1–5). These disorders combine cognitive (primarily attention, speed of information processing, and learning efficiency), motor, and behavioral symptoms, which suggest that the virus preferentially targets the subcortical white matter. Neuropathologic studies have shown histopathologic changes in the subcortical white matter, including multinucleated giant cells, astrogliosis, and myelin pallor (3, 4, 14–16).
Recent evidence suggests that elevations in the CNS viral load are associated with HIV-related neurocognitive disorders (16–19). Further, there is evidence of both genotypic and phenotypic differences in the strains of HIV detected in the CNS compared with those in the peripheral circulation (17). This evidence supports the concept that the CNS is an independent “reservoir” or “sanctuary” for HIV, particularly in the later stages of the disease when neuropsychiatric disorders are most likely to occur (20). The concept that the CNS acts as a reservoir for HIV is important relative to antiretroviral therapy, because sufficient blood brain–barrier penetration would be needed if antiretroviral therapy were to be used to treat cognitive disorders (21).
The recent development of protease inhibitors has significantly decreased disease progression and mortality (5). Combination therapy, which uses both nucleoside analog reverse transcriptase inhibitors, such as azidothymidine, and protease inhibitors, has currently become the treatment of choice for patients with HIV. During the past year, reports have emerged showing that patients receiving HAART have experienced reductions in CSF viral load levels (22, 23), reversal of white matter lesions on MR images (5), and improvements in neuropsychological test performance (24). However, other studies have documented progressive neurologic impairment despite the use of HAART and despite low viral load levels (25). Because most currently available protease inhibitors, with the exception of indinavir, do not have good blood brain–barrier penetration, it raises concerns that despite improvements in general health, patients receiving HAART will continue to experience neurocognitive decline (26). Because the CNS is likely a reservoir site for HIV, this could allow for the development of viral resistance and reseeding the peripheral circulation with drug-resistant virus.
The development of a neuroimaging marker for HIV-associated neuropathologic changes in the CNS may allow for the earliest possible detection of cognitive impairment, and, in theory, it could be used to measure response to HAART. In this study, we showed that abnormalities in the subcortical white matter and corpus callosum on MR diffusion tensor images could be detected for patients with HIV who had normal-appearing white matter on their MR images of the brain. The largest decreases in anisotropy in the corpus callosum and the largest elevations in the diffusion constant (Dav) in the subcortical white matter occurred in patients who had the most advanced HIV disease with the highest viral load levels and lowest CD4 counts (Fig 2 [page 279]). In this set of patients, the Dav of the splenium was significantly elevated (P < .02) and the anisotropy of the genu (P < .002) and splenium (P < .0004) were both significantly decreased. The patients with HIV who had nearly normal Dav and normal anisotropy had the lowest peripheral viral load levels and highest CD4 counts, on average. Patients with intermediate viral load levels (10,000−200,000 copies of HIV messenger RNA) had elevations in the Dav of 8% to 12% in the subcortical white matter but statistically significant decreases in anisotropy of 17% to 18% (P < .02 for genu, P < .02 for splenium) compared with the group of patients whose values were normal. In general, as viral load levels rise and CD4 counts decline, these patients had increases in the Dav and declines in the anisotropy. Thus, the diffusion constant (Dav) and diffusion anisotropy may be useful neuroimaging markers for HIV disease.
Although the four patients with intermediate viral load levels had the highest mean CD4 count of 522, this reflects that one of these patients, who had early HIV disease (stage A1), had a relatively high peripheral viral load (35,000 copies of HIV messenger RNA/mm3) and a very high CD4 count of 1536.0. If this datum point were not included, the mean CD4 count for these patients would be 183.0, which is significantly lower compared with the mean CD4 count of patients with the lowest viral load levels, who would be expected to have higher CD4 counts. Even with the inclusion of this datum point, as CD4 counts decline, there is a corresponding rise in the diffusion constant and decline in anisotropy.
In most of the patients with HIV, there was a greater elevation in the Dav in the subcortical white matter of the frontal lobe region when compared with the parietooccipital subcortical white matter, which may reflect a predilection for frontal white matter involvement in direct HIV infection in the CNS. In diffusion MR imaging, a loss of normal cell membrane homeostasis, which changes the net translational movement of water across cell membranes, has been hypothesized to create the signal abnormalities observed on MR images (27, 28), This can be quantified from the ADC maps, and this value, the diffusion constant or Dav, can reveal changes in white matter that are not seen on routine MR images of the brain (27, 28). In HIV-associated dementia or mild cognitive motor disorder, the subcortical white matter is preferentially affected (1–4, 14–16), and this may explain the changes in the diffusion constant that we observed in the subcortical white matter. In both these groups of patients, the Dav of the centrum semiovale was normal, which may signify that this area of white matter is affected later by HIV or may be more resistant to HIV infection.
With routine diffusion-weighted imaging, one assesses overall changes in the degree of diffusion, which intentionally eliminates the effects of tissue anisotropy (8). However, tissue microstructure is affected by the motion of water molecules on this scale, which determines the degree of anisotropy (8). One can acquire diffusion-sensitive (diffusion tensor) images in which this directional information is measured. By determining the anisotropy of white matter fiber tracts, which are highly ordered and have distinct directions, one can make inferences regarding the integrity of white matter fiber microstructure (8).
In this study, both the patients with intermediate and high viral load levels had statistically significant decreases in the measured anisotropy of the genu and splenium of the corpus callosum. The decreases in anisotropy that we have observed in this study would imply microscopic damage to these fiber tracts despite their normal appearance on macroscopic MR images of the brain. This may be explained in that HIV-associated dementia and mild cognitive motor disorder are thought to be disease processes in which there is preferential involvement of the white matter (1–4, 14–16).
Patients with undetectable viral load levels who were receiving HAART had normal anisotropy in both the genu and splenium of the corpus callosum. However, the diffusion constant was not normal in the patients with undetectable viral load levels. There was an elevation in the diffusion constant in the genu, which implies lack of restriction to water translation across this white matter fiber tract. This may simply represent an increase in the amount of water in the extracellular compartment despite normal-appearing T2-weighted and fluid-attenuated inversion recovery images. One could speculate that this represents myelin damage, which occurs in association with HIV infection in the CNS, because the myelin shows pallor on autopsy studies (15).
In patients with undetectable viral load levels, the splenium of the corpus callosum showed an apparent decrease in the diffusion constant to 0.76 × 10−5cm2/s. This value could be within the lower limits of normal, because the normative data for the Dav of the splenium was 0.88 × 10−5cm2/s ± 0.10 and the SD of this measurement shows a relatively wider range of variability for this measurement as compared with others. If this represents a real decrease in the measured Dav, more restriction to the normal translation of water in the splenium is implicated. The reason for this observation is unclear. Histopathologic studies of HIV infection of the brain have shown that there is an inflammatory response initiated early in the course of the disease (15). In theory, this inflammatory response could create a local hypercellular environment, which could impose more restrictions than normal to water diffusion. In general, for these patients, as viral load increased, there was a corresponding increase in the diffusion constant and decrease in anisotropy within the splenium.
Three of four patients with intermediate viral load levels and one of the patients with the highest viral load levels were not receiving HAART. All of these patients had marked elevations in the Dav in all locations measured and statistically significant decreases in the anisotropy of the genu and splenium despite normal-appearing white matter on MR images. Conversely, the patients with normal values who had the lowest viral load levels were all receiving HAART. It is not clear whether HAART can reverse abnormalities in the Dav or anisotropy. However, it seems that those patients who receive HAART have healthier white matter, because these patients have normal diffusion constants and anisotropy values indicative of normal translation of water molecules across myelin membranes, which implies integrity to the underlying white matter fiber tracts. To determine the effect of HAART on the diffusion constant and anisotropy of white matter fiber tracts, which are targeted by the virus when it gains access to the CNS, it will be important to study protease inhibitor–naïve patients who are going to begin HAART. A longitudinal, prospective study, which correlates CSF viral load levels, neuropsychological testing, quantification of drug levels, and MR diffusion tensor imaging findings may help to determine whether MR diffusion tensor imaging can act as a neuroimaging outcome measure of HAART efficacy.
Conclusion
In summary, in patients who have tested positive for HIV who have normal-appearing white matter on MR images of the brain, calculating the diffusion constant and anisotropy can detect abnormalities within the subcortical white matter and corpus callosum. Patients who had the highest elevations in the diffusion constant and the largest decreases in anisotropy had the most advanced HIV disease, as manifested by high peripheral viral load levels and low CD4 counts. Furthermore, most of these patients were not receiving HAART. Because diffusion tensor MR imaging can detect abnormalities missed by routine MR imaging, MR diffusion tensor imaging may have a role as a marker of HIV disease progression or HAART efficacy.
Footnotes
↵1 Address reprint requests to Christopher G. Filippi, MD, New York Presbyterian Hospital-Weill Medical College of Cornell University, Department of Radiology, Box 141, 525 East 68th Street, New York, NY 10021.
References
- Received February 8, 2000.
- Copyright © American Society of Neuroradiology