Abstract
Summary: We report the use of diffusion-weighted MR imaging in the early diagnosis and monitoring of the progression of a histopathologically proved case of sporadic Creutzfeldt-Jakob disease. Ribbon-like areas of hyperintensity in the cerebral cortex on diffusion-weighted images corresponded to the localization of periodic sharp-wave complexes on the electroencephalogram.
Sporadic Creutzfeldt-Jakob disease (CJD) is a rare fatal dementing illness caused by a prion agent composed of protease-resistant protein (1). Antemortem diagnosis remains problematic because the characteristic diagnostic triad of progressive dementia, myoclonic jerks, and periodic sharp-wave electroencephalographic (EEG) activity may be lacking in as many as 25% of these patients. (2). Routine MR imaging sequences (T1-weighted, T2-weighted, fluid-attenuated inversion recovery [FLAIR], gadolinum-enhanced T1-weighted, and gradient-echo sequences) may show abnormalities in the basal ganglia and cerebral cortex; however, 21% of patients with sporadic CJD have normal MR imaging findings (3–5). During the last 5 years, several reported cases of sporadic CJD have shown increased signal intensity in the basal ganglia and/or the cerebral cortex on diffusion-weighted (DW) images; this finding is indicative of reduced diffusion (Table) (6–13).
Review of pathologically proved CJD cases with positive DW imaging findings reported in the literature
We report a case of sporadic CJD proved with brain biopsy. We compared the evolution of the clinical features, DW imaging abnormalities, and EEG findings to assess the usefulness of DW imaging in establishing the diagnosis and following the progression of the disease. In addition, we review the literature on the correlation between DW imaging and routine MR imaging findings and the clinical, electrophysiologic, and histopathologic features of sporadic CJD.
Case Report
The patient was a 63-year-old right-handed man with a history of controlled hypertension and hypercholesterolemia. He was a retired mechanic and an avocational carpenter and handyman. Six months before presentation, his wife noted his forgetfulness, a need for him to repeat carpentry measurements, and an inability or lack of desire to complete tasks. He developed generalized fatigue, decreased appetite, and disruption of sleep due to involuntary leg movements. He experienced episodes of disorientation and confusion, becoming lost in his own kitchen and while driving in a familiar neighborhood. He became impotent. Three months before presentation, he developed a significant decrease in spontaneous speech and was incapable of engaging in a conversation. There was an episode of loss of balance with a fall to the floor. His Folstein Mini-Mental State Examination (MMSE) score at that time was 29 of 30. He was diagnosed with depression (pseudodementia), and citalopram hydrobromide 20 mg/day was started. Three weeks before presentation, he lost the ability to read a newspaper. Two weeks before presentation, he stopped acknowledging his wife’s name and began to require assistance for activities of daily living.
At the time of presentation, he knew his name but was disoriented to place, time, and identification of other people. He could slowly repeat simple sentences, name simple high-frequency subjects, and follow one-step commands. He could not read, write, spell, calculate, or construct. His MMSE score was 3 of 30. More detailed neuropsychiatric testing was attempted but could not be accomplished. The remainder of the neurologic and systemic examination findings were normal. No involuntary movements were observed. Review of medical systems, travel history, and family history were noncontributory. The patient did not smoke and did not drink alcohol. He had a remote history of occupational exposure to mercury vapors. Extensive serum and urine laboratory evaluations were normal. CSF studies including 14-3-3 protein were also normal.
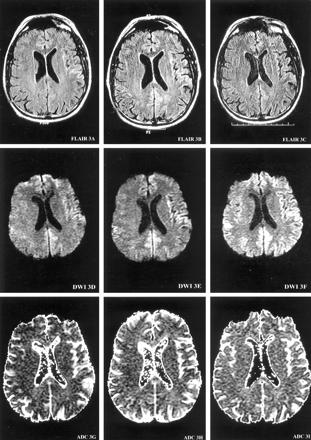
MR imaging of the brain with a DW sequence (9999/98.8 [TR/TE], 5-mm-thick sections, diffusion gradient strength 1000 s/mm2) was performed at 4, 5.5, and 6 months after the onset of symptoms (Fig 1). T1-weighted (566/11.3, 2-ms echo train length [ETL], T2-weighted (4000/102, 16-ms ETL), gadolinium-enhanced T1-weighted (566/11.3, 2-ms ETL), and gradient-echo (750/35) images were consistently normal. At 4 months from onset, DW images demonstrated increased signal intensity in the right temporal cortex in a gyriform pattern. At 5.5 months from onset, the areas of hyperintense signal on DW images involved more cortical gyri and extended to the left temporoparietal cortex. At 6 months from onset, DW images showed a ribbon-like area of hyperintensity involving the right temporoparietal occipital cortex, as well as the left parietal and frontotemporal cortex extending into the parafalcine occipital region (Fig 1). Qualitative apparent diffusion coefficient (ADC) mapping of these regions revealed a decreased signal intensity indicating the presence of restricted diffusion. FLAIR images (10,002/174/2200 [TR/TE/TI]) at 6 months showed subtle hyperintense signal in the left frontotemporal region, corresponding to the observed DW imaging findings (Fig 1). MR proton spectroscopy was performed at 6 months from symptom onset and revealed a slightly decreased ratio of the peaks for N-acetylaspartate to creatine (1.46–1.56 [normal >1.8]) measured in the parafalcine occipital and left frontal cortical regions.
Serial MR images demonstrate the evolution of ribbon-like cortical signal intensity abnormalities. MR images are presented at each of three brain axial levels (1–3), at each of three time points from the onset of symptoms (left columns, 4 months; middle columns, 5.5 months; right columns, 6 months) for FLAIR (top row), DW imaging (middle row), and ADC (bottom row) studies. At 4 months from onset of symptoms, DW images demonstrate gyriform increased signal intensity predominantly in the right temporal cortex (DWI1D) with decreased ADC signal intensity consistent with restricted diffusion (ADC1G). At 5.5 months from onset, the hyperintense signals on DW images involve more cortical gyri, extending into the left temporoparietal cortex (DWI3E). At 6 months from onset, DW images (DWI1F, DWI2F, and DWI3F) show ribbon-like areas of hyperintensity involving the right temporoparietooccipital cortex, as well as the left frontotempoparietal cortex, extending into the parafalcine occipital region.
Images on this page were obtained at level 1.
(continued) Images on this page were obtained at level 2.
(continued) Images on this page were obtained at level 3.
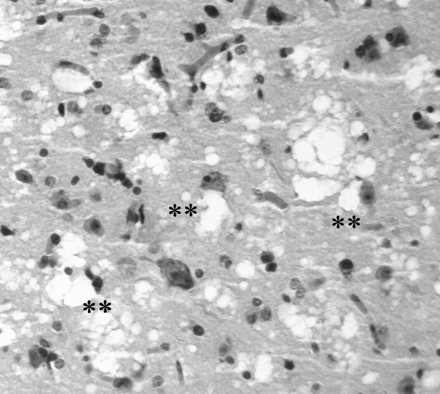
EEGs at 6 months from onset of symptoms showed diffusely slow and disorganized waking cerebral background activity and left frontotemporal periodic sharp-wave complex discharges (Fig 2), which corresponded to the hyperintense signals on DW images. Left frontal brain biopsy demonstrated gray matter spongiform degeneration with characteristic intracytoplasmic vacuoles, which are the primary pathologic features of sporadic CJD. Neuronal loss was prominent. Astrocytosis was not conspicuous (Fig 3).
A 21-channel EEG with the patient awake demonstrates periodic sharp-wave complex discharges, which are of greater amplitude over the left frontotemporal region and are superimposed on diffusely disorganized and slow background.
Photomicrograph of brain biopsy specimen obtained from the cortex of the left frontal lobe demonstrates prominent neuronal loss. Astrocytosis is not conspicuous. There are characteristic spongiform changes (**) that tend to be cell-associated (hematoxylin-eosin stain, original magnification ×400).
The patient continued to decline and died 8 months after the onset of symptoms. Brain autopsy was performed and demonstrated diffuse gray matter spongiform degeneration. Immunohistochemical staining (primary antiserum: polyclonal goat antihuman, AHP498; Serotec, Raleigh, NC) for human prion protein (CD230) deposition was positive, which further confirmed the diagnosis of sporadic CJD (data not shown).
Discussion
CJD may present as a sporadic CJD or familial CJD disorder. It is one of several spongiform encephalopathies that also include kuru, Gerstmann-Sträussler-Scheinker disease, familial fatal insomnia, and new variant CJD (14). The worldwide incidence of sporadic CJD is approximately one case per million population per year (1). Sporadic CJD is characterized by rapidly progressive dementia, myoclonus, and ataxia (2) and is typically fatal within 1 year of onset of symptoms (1). Although the diagnosis of sporadic CJD may be suspected on the basis of the clinical presentation, confirmation of the diagnosis rests on obtaining brain tissue for pathologic examination either by brain biopsy or at autopsy.
Several CSF markers of neurodegeneration have been used to support the clinical diagnosis of CJD, including neuron-specific enolase, S-100, and/or 14-3-3 protein. Of these markers, 14-3-3 protein has good sensitivity (90–96%) and the highest specificity (93–100%) (15–18). Periodic sharp-wave complex discharges are observed on EEGs in as many as 67% of patients with sporadic CJD (3, 19), but these changes may not develop until late in the course of the disease (20) and may disappear as the disease progresses (21). Repetitive EEG monitoring may increase the test sensitivity to 90% with specificity as high as 86% (19, 22). Routine brain imaging methods, including CT, have not been proved useful in the diagnosis of sporadic CJD, as nonspecific atrophy may be present late in the disease course (1). Although the specificity of routine MR imaging for sporadic CJD may be as high as 93% (4), the sensitivity in detecting abnormalities is only 67–79% (4, 23). When observed, the abnormalities generally appear late in the course of disease, thus limiting the diagnostic value of routine MR imaging (4). In patients with advanced CJD, MR proton spectroscopy has demonstrated decreased concentration of the neuronal marker N-acetylaspartate, which is a nonspecific finding (24).
In our case, sporadic CJD was suspected owing to the patient’s rapidly progressive dementia; however, an unequivocal clinical diagnosis was complicated by the absence of myoclonic jerks or ataxia and the presence of normal levels of 14-3-3 protein in the CSF. Brain CT and routine MR imaging (except for subtle FLAIR findings at 6 months) were also unrevealing in our patient. DW images did show marked changes beginning as early as 4 months from the onset of behavioral symptoms when the MMSE was nearly intact. These DW imaging findings progressed over 2 months (Fig 1), coincident with the deterioration of the patient’s MMSE score and independence for performing activities of daily living. At 6 months of illness, EEG revealed periodic sharp-wave complex discharges over the left frontotemporal cortex that correlated with DW imaging changes (Fig 2). Notably, EEG did not demonstrate similar changes in the right temporal cortex where comparable DW imaging abnormalities were also seen (Figs 1 and 2). Therefore, in this patient, the distinctive changes in the ribbon of the cerebral cortex on DW images were the earliest laboratory studies to provide support for the diagnosis of CJD. These DW imaging abnormalities appeared in advance of a deterioration of the MMSE score and in the absence of elevations of CSF 14-3-3 protein levels.
Distinctive patterns of hyperintensities in the basal ganglia and/or in the cerebral cortex on DW images have been reported in 17 cases (including the present case) of pathologically proved sporadic CJD (Table). In four of these 17 cases, no abnormalities were seen on routine MR images; nine showed subtle changes on FLAIR and/or T2-weighted images; only four clearly showed hyperinternse signals on FLAIR and/or T2-weighted images. EEGs were recorded in six of these 17 cases and showed changes (eg, periodic sharp-wave complex discharges) in only three cases (50%). CSF assays (14-3-3 protein or neuron-specific enolase) were performed in nine of these 17 cases, of which seven (78%) were positive. In addition to these 17 cases, eight cases of progressive dementia with comparable DW imaging findings and supportive CSF and/or EEG findings were presumed to be sporadic CJD without pathologic confirmation (25–29). Two cases of progressive dementia were reported as familial CJD based on gene mutational analyses and family history; these cases also demonstrated similar DW imaging changes, but no pathologic examinations were undertaken for confirmation (30).
Although it is unclear whether DW imaging abnormalities are seen more reliably in early CJD than are changes with other diagnostic modalities, it is notable that such DW imaging changes have been observed as early as 1 month after the onset of symptoms (Table). Whether DW imaging abnormalities are pathognomonic of sporadic or familial CJD, or of spongiform encephalopathies in general, is also unclear. The pathologic basis for DW imaging changes in the basal ganglia and cerebral cortex in CJD is not well understood. Spongiform neuronal degeneration may contribute to changes detected with DW imaging by altering the molecular motion of water (31). ADC mapping abnormalities have been reported in CJD (7–10, 24); however, since both increased and decreased signal intensities have been observed on ADC maps at approximately 1–1.5 months from the onset of symptoms (9, 24), it is less likely that ADC mapping variations signify the stage of the illness. High-signal-intensity regions observed on DW images in sporadic CJD have correlated with areas of decreased perfusion on SPECT scans (6) and with areas of hypometabolism on PET scans (9).
Antemortem diagnosis of CJD is often complicated by the need for brain biopsy. Handling of CJD brain tissue may place hospital personnel at risk (1). By contrast, DW imaging is a noninvasive and extremely rapid technique (imaging times are typically less than 30 seconds). Rapidity is particularly advantageous for CJD patients who may have prominent myoclonus or who are poorly cooperative with procedures. DW imaging patterns of ribbon-like abnormalities in cerebral cortex and/or hyperintensities in basal ganglia may offer the means for diagnosis of CJD early in the course of the illness and for monitoring disease progression (Fig 1) (9, 26, 28). Abnormal DW imaging changes may be present in patients with CJD who have normal EEGs (7, 8, 13) and normal CSF studies (8, 26). Further prospective studies of DW imaging in patients being evaluated for possible CJD should clarify the diagnostic utility of this imaging technique.
Acknowledgments
The authors thank Drs Rup Tandan and Jeffrey Florman for thoughtful contributions to the manuscript. We thank Dietrich Schultze and Adam Riesner for their assistance in preparing the images.
References
- Received May 22, 2001.
- Accepted after revision September 26, 2001.
- Copyright © American Society of Neuroradiology