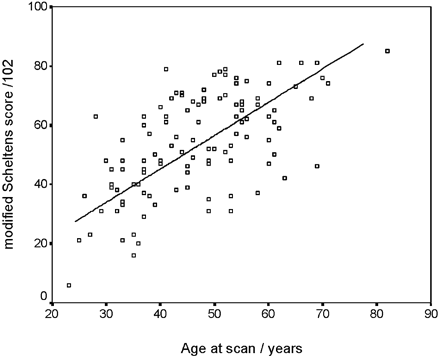Abstract
BACKGROUND AND PURPOSE: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a condition causing recurrent subcortical strokes. MR imaging, which shows focal lacunar infarcts and leukoaraiosis, plays a central role in the diagnosis and evaluation. We studied MR imaging abnormalities in a large prospectively recruited cohort of CADASIL patients to describe the spatial distribution of abnormalities, determine how this distribution alters with age, and identify any correlations with the clinical features of the disease.
METHODS: In this study, 112 CADASIL subjects from 64 families were prospectively recruited. MR imaging scans were graded by a single neuroradiologist, by using the modified Scheltens scale, to quantify the severity of high-signal-intensity changes in different brain regions.
RESULTS: Lesion load increased progressively with age. Scores were maximal in the frontal, parietal, and anterior temporal cortex, and the external capsule; intermediate in the pons; and relatively low in the corpus callosum, caudate, globus pallidus, cerebellum, midbrain, and medulla. Anterior temporal pole involvement was common at all ages and, when present, usually confluent, but this was absent in 33% of patients 20–29 years of age. A history of stroke correlated with total Scheltens score and internal capsule and pontine scores. Dementia correlated with total Scheltens score and subcortical white matter score, whereas depression correlated with subcortical white matter score but not total Scheltens score.
CONCLUSIONS: There is a characteristic pattern of MR imaging abnormalities in CADASIL that aids in differential diagnosis; however, some characteristic features, such as anterior temporal pole involvement, can be absent. MR imaging lesion load correlated with some clinical features including stroke and dementia, whereas depression is more common in individuals with deep white matter changes.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an autosomal dominant condition causing recurrent subcortical strokes, often complicated by a subcortical dementia. Migraine with aura and depression are other common features. The condition is caused by mutations in the notch3 gene (1). Neuroimaging shows both focal lacunar infarcts and diffuse white matter ischemic changes known as leukoaraiosis. MR imaging plays a central role in the diagnosis and evaluation of patients with CADASIL. Despite its importance, there have been few systematic MR imaging studies of large series of patients with the disease (2–5). Leukoaraiosis is a nonspecific term originally coined to describe the radiologic appearance of rarefaction of the white matter on CT (6), which is also seen as corresponding high signal intensity on T2-weighted MR imaging. It has a wide differential diagnosis. In particular CADASIL may be misdiagnosed as multiple sclerosis (7).
Initial studies suggested the MR imaging pattern was similar to that seen in patients with sporadic small-vessel disease, but more recent studies have demonstrated significant differences, particularly involvement of the anterior temporal pole, external capsule, and corpus callosum (8, 9), but there are limited data on how often these abnormalities are seen in a large population and when they first appear. Therefore, we studied the spatial distribution of MR imaging abnormalities in a large prospectively recruited cohort of CADASIL patients. We hypothesized that there would be a typical pattern with preferential involvement of particular areas. We also determined how this distribution altered with age and whether there were any correlations with clinical features of the disease.
Methods
Subjects
We prospectively recruited 112 CADASIL patients from 64 families as part of a British CADASIL prevalence study. Potential symptomatic cases were identified via the British Neurologic Surveillance Unit and then reviewed in person at our center or visited in their home by the research team. Unaffected family members were then invited to attend for clinical assessment, MR imaging, and genetic testing. Diagnosis was made by detection of a pathogenic notch3 mutation on direct sequencing (108 cases), the presence of characteristic granular osmiophilic material (GOM) on electron microscopy of a skin biopsy in combination with a characteristic clinical history (2 cases), and by characteristic MR imaging appearances showing white matter high signal intensity in a first-degree relative with biopsy-confirmed CADASIL (2 cases). MR imaging scans were acquired as part of routine clinical evaluation in individual centers throughout the Great Britain, and therefore standard clinical protocols were used on a number of different systems. T2-weighted or, in 6 cases, proton density acquisitions were used for analysis. Where fluid-attenuated inversion recovery (FLAIR) images were also available, they were used to help distinguish the presence of a true lesion from CSF, but all scores were still based on the proton density/T2-weighted images.
In addition, MR imaging scans with both T2 and FLAIR sequences were also obtained on relatives of affected patients who had not been clinically tested but had given written informed consent to be scanned for research purposes only. They were obtained on a 1.5T GE Signa system (Milwaukee, WI). Both a dual spin-echo TE 17/102 msec, TR 4000 msec, providing T2 and proton density images, and an axial FLAIR sequence (TE 135 msec, TR 9500 msec) were performed. Multiple contiguous 3-mm sections were prescribed in the true axial plane to provide complete brain coverage. In-plane resolution was 0.86 mm × 0.86 mm.
MR Imaging Lesion Scoring
Original scans were rated by using a modified version of the semiquantitative Scheltens scale by the same consultant neuroradiologist (P.R.). This is a validated scale with high intra- and interobserver reliabilities, originally designed for the purpose of measuring spatial distribution of periventricular white matter, basal ganglia (BG), and infratentorial (IT) lesions (10). It provides separate information about white matter, BG structures, and IT regions. Each region is scored on a scale of 6, depending first on size and then on number of lesions. For example, the presence of any lesion of 4 mm or larger scores at least 3 and confluence of lesions scores 6. Scores of 1–2 were considered to represent minor change, scores of 3–4 moderate change, and scores of 5–6 severe change. Confluence was defined as an area of relatively continuous signal intensity change >10 mm in size.
Modifications were made to ensure the scale was sensitive to regions previously reported as being particularly involved in CADASIL. To identify anterior temporal lobe involvement, the temporal lobe was divided into anterior and posterior lobes, by using the posterior border of the amygdala. The external capsule was also scored. A high inter-rater reliability for these modifications has been demonstrated elsewhere (9). Corpus callosum involvement has been reported in CADASIL (4); involvement of this structure is characteristic of multiple sclerosis, a common differential of CADASIL, and is rare in sporadic cerebral small-vessel disease. Therefore, this structure was also scored.
Clinical Outcome Scales
All patients were reviewed clinically and details of previous transient ischemic attack (TIA) and stroke, dementia, migraine, primary epilepsy, acute encephalopathy, and psychiatric disorder were recorded. Dementia was defined as either a previous diagnosis made by a specialist or a Mini-Mental State Examination (MMSE) score of <23 in the presence of unimpaired consciousness, the latter cutoff being indicative of DSM-R-III–and ICD-10–defined dementia (11). Primary epilepsy was defined as adult-onset seizures not associated with acute stroke or encephalopathy. Acute reversible encephalopathy was defined as a transient episode of subacute onset of impaired consciousness with no apparent cause and requiring hospital admission (12). Psychiatric disorder was defined as a diagnosed psychiatric syndrome requiring drug treatment. To assess physical disability, we used the modified Rankin score. This is a validated scale commonly used to assess handicap in stroke patients (13). Scores are integers ranging from 0 (asymptomatic) to 5 (24-hour nursing care). Scores <3 indicate independent functioning, and patients were divided into independent or dependent groups by using this cutoff.
Data Analysis and Statistics
The statistics software package SPSS (SPSS, Chicago, IL) was used for all calculations. Comparisons between group means were carried out by using the t statistic for parametric data and Spearman ρ statistic for nonparametric data. Logistic regression was used to assess the risk of a clinical outcome conferred by an increase in Scheltens score.
Results
Mean age was 47 years (range, 23–82 years). The numbers of subjects in each decade were 20–29 years, 6; 30–39 years, 28; 40–49 years, 32; 50–59 years, 28; 60–69 years, 15; 70–79 years, 2; 80–89 years, 1; 47 (42%) were male. Three subjects were entirely asymptomatic; 62 (55%) had suffered stroke or TIA; 84(75%) suffered from migraine, usually with aura; 6 (5%) had primary epilepsy; and 13 (12%) had experienced an acute reversible encephalopathic episode. Thirty-five (31%) had a diagnosed psychiatric disorder, usually depression, and in 25 of them this had preceded the onset of stroke or dementia; 22 (20%) had a diagnosis of dementia; and 25 (22%) had migraine alone.
Mean (SD) total Scheltens score was 54 (17) with a range of 6–85. Total Scheltens score increased with age (R = 0.619; P < .001), though after the age of 50 years the rate of progression slowed. There was, however, a wide intersubject variation in total scores in any particular age group (Fig 1). The spatial distribution of scores across all regions is shown in Table 1. The median score was 6 (maximal) for the following regions: frontal, parietal, anterior temporal, and external capsule. The following regions had median scores of 0 or 1, reflecting relatively limited involvement: corpus callosum, caudate, globus pallidus, cerebellum, midbrain, medulla. Of the IT regions, only the pons showed moderate involvement, with a median score of 3 and a mean score of 3.5.
Relationship between total Scheltens score and age.
Scheltens scores for individual regions
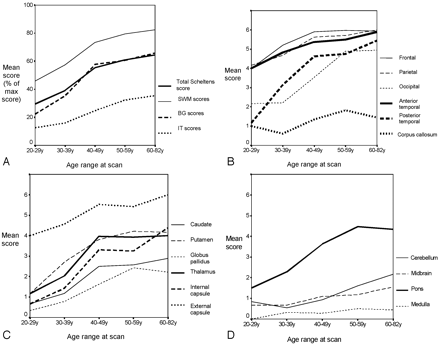
Differences in the spatial distribution across different age ranges is shown in Figs 2A–D. Scores for subcortical white matter (SWM) and BG structures increased approximately linearly until the fourth decade, after which the level of increase leveled off (Fig 2A). The IT structures were less involved, but there was a progressive increase in scores with age across all age groups (Fig 2A).
Mean scores by age for different brain regions. A, Composite scores of total and major regions. B, Cortical regions. C, BG structures. D, Other infratentorial structures. The y axis represents the total possible score. In panel A, where this differs for different regions the percentage of maximum possible score for each regions is given. In panels B–D, where the maximum score for each region is 6, the mean score is given for each region.
Figure 2B shows changes in lesion load in different SWM regions with age. It demonstrates a high level of involvement of the frontal, parietal, and anterior temporal regions even in the younger age groups, with progressive increase up to the fourth decade before a leveling off. In contrast, involvement of the posterior temporal and occipital white matter was less marked in the younger age groups, but increased markedly to be maximal in the 60–82-year-old age group. Involvement of the corpus callosum was limited at all ages and showed only a slight increase with age.
Figure 2C shows involvement of the BG structures, including the internal and external capsule, across the different age groups. This demonstrates preferential involvement of the external capsule with a mean score of 4, even in the youngest age group, and a progressive increase to a mean score of almost 6 in the older subjects. In contrast, involvement of the other BG regions is limited in younger individuals, but then progressively increases with age.
Figure 2D shows involvement of the IT structures. This illustrates preferential involvement of the pons with a progressive increase in lesion load until the 50–59-year-old age range after which this levels off. Cerebellar involvement is unusual up to age 40, but after this progressively increases. Midbrain lesion load is low at all ages, but there is a slight increase with age. Medulla lesion load is low at all ages.
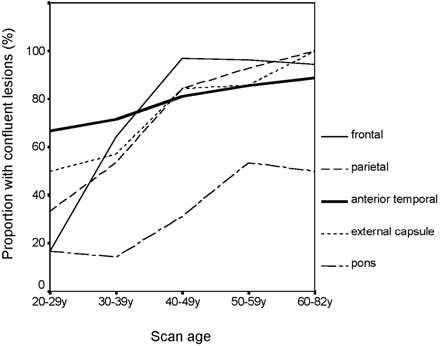
The distribution was examined in more detail across age for those regions most often involved. Moderate involvement (defined as Scheltens score ≥3) was present in >80% of parietal regions and in >60% of frontal, anterior temporal, and external capsular regions in all age groups, even the youngest. In contrast, moderate pontine involvement was unusual in young patients and was not seen in >60% until the 40–49-year-old age group. In the 6 patients aged 20–29 years, the most frequently affected regions were frontal (100%), parietal (100%), external capsule (83%), and anterior temporal (67%). The median scores for these 4 regions in this age group were frontal, 4.5; parietal, 4.5; external capsule, 5.5; and anterior temporal, 6. This reflected the fact that, although anterior temporal pole involvement was not always present in the youngest age group, when it was present changes were usually confluent. Although confluent anterior temporal pole involvement was a characteristic feature, it was absent in a small proportion of the 60–82-year-old age group (Fig 3). In contrast, in this age range, all subjects had confluent external capsule lesions. Illustrative MR imaging scans with case histories are shown in Figs 4⇓–6.
Proportion of confluent lesions by age.
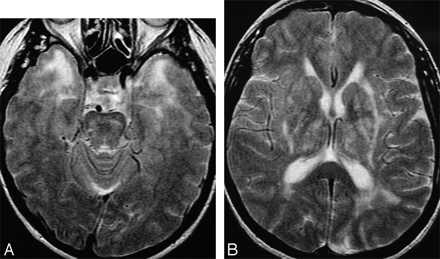
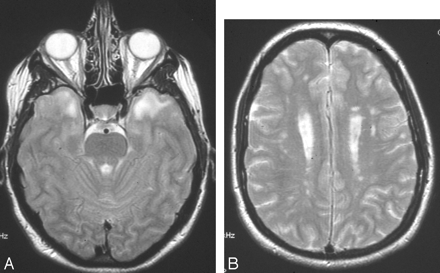
The characteristic changes in CADASIL in (A) anterior temporal change (graded 6 on the Scheltens scale) and (B) involvement of the external capsule (grade 6). There is also confluent (grade 6) involvement of parietal white matter on this section.
MR imaging from a 33-year-old woman who had experienced classical migraine with aura since her teens, but was otherwise asymptomatic It shows how marked anterior temporal pole involvement (grade 6) (A) can occur in the presence of much lesser involvement in other white matter structures. In panel B, frontal white matter was graded as 3 and parietal as 2.
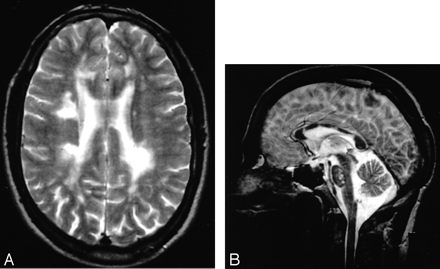
This MR imaging illustrates involvement of the corpus callosum. This is seen on the axial scan (A), where it was graded as 3. It is better seen on the sagittal scan (B), which also shows pontine involvement.
Correlations with Clinical Features
There was a highly significant correlation between all 16 individual regions scored and age (P < .002 for all), except for the anterior temporal lobe (ρ = 0.173; P = .068) and the medulla (ρ = 0.106; P = .266). Therefore, all correlations between clinical parameters and individual regions are adjusted for age.
A past history of stroke correlated with total Scheltens score (P = .005). Study of individual regions revealed that a history of stroke was independently correlated with both internal capsular (P = .025) and pontine scores (P = .011).
There was no correlation between migraine and involvement of any specific regions. Psychiatric disorder (in most cases, depression) correlated with SWM score (P = .044), but not with total Scheltens score (P = .363). A diagnosis of dementia correlated with total Scheltens score (P = .022) and SWM scores (P = .005). There was no correlation between global or any regional scores and reversible encephalopathy or primary epilepsy.
Disability was assessed by using the Rankin score. Rankin score was associated with age, and after covarying for age with total Scheltens score (P = .023). This was no longer significant after covarying for history of stroke (P = .06).
Discussion
In this large, prospectively identified cohort of CADASIL patients, we have described the spatial distribution of abnormalities on MR imaging and how this distribution changes with age. Our results confirm the high sensitivity of MR imaging even in young patients with CADASIL. They also show the preferential involvement, and different rates of evolution, of disease in different regions. These results, from the largest published series of MR imaging in patients with CADASIL, provide a reference for neurologists and radiologists to use when considering whether an MR imaging scan with subcortical high signal intensity is consistent with a diagnosis of CADASIL.
Consistent with previous studies, our results demonstrate that in younger patients the most commonly affected regions are the frontal, parietal, and anterior temporal white matter and the external capsule (2, 4, 5, 8, 9, 14). In these regions, there is a progressive increase in Scheltens score until the fifth decade, after which the rise appears to level off. This finding has important implications for early diagnosis of the disease. Previous studies have suggested that both anterior temporal lobe and external capsule involvement are useful markers that may allow differentiation from other forms of small-vessel disease (8, 9). A more recent study has suggested that anterior temporal involvement has a much higher specificity than external capsule involvement (15), and in 40 individuals it was reported in all cases, even in 5 in the third decade (5). Our results show that moderate or severe anterior temporal pole involvement is common in more than half of individuals in the youngest age range, but that it can be completely absent in a minority of individuals at this age. The leveling out of Scheltens score with age in the more severely affected regions is likely to reflect the semiquantitative nature of this scale with a ceiling effect. A maximal score of 6 can represent both early and late confluent disease.
Other regions show only moderate involvement. These tend to be less involved at presentation, but lesion load then progressively increases across all age ranges. These regions are the posterior temporal and occipital white matter, the BG nuclei, thalamus and internal capsule, and the pons. In contrast, some regions are much less involved in CADASIL. These include the corpus callosum and all IT regions except the pons. Corpus callosum involvement is of particular diagnostic importance. This is a characteristic feature of multiple sclerosis but is rare in sporadic forms of small-vessel disease. For this reason, it has been used to differentiate between demyelination and ischemia. In patients with CADASIL, in contrast to sporadic small-vessel disease, it has been highlighted as a common finding, occurring in 5 of 8 affected individuals from a single family (4). This could lead to misdiagnosis of CADASIL as multiple sclerosis. Our findings show that callosal involvement on axial scans does occur in a minority of patients with CADASIL but is less frequent than involvement of many other regions. It is likely that, on sagittal scans, callosal involvement may be more frequently detected.
The major differential diagnosis of confluent white matter high signal intensity on MR imaging, apart from demyelination, is vascular disease. Most common is sporadic small-vessel disease, for which the major risk factor is hypertension, though anterior temporal pole involvement is rare (9). Other, rarer causes include cerebral vasculitis—particularly primary angiitis—leukodystrophies, infective conditions such as Lyme disease, and neurosarcoidosis. Rarer genetic causes include Fabry disease and mitochondrial disease. Internal watershed ischemia due to carotid artery disease can also result in deep white matter high signal intensity, though this is usually unilateral.
A previous study of 64 patients with CADASIL found a significant correlation between quantitative T2 lesion volume and cognition, as assessed by the MMSE (16). This study did not, however, look at associations between specific regions and cognitive and clinical features. There have been limited studies examining these associations, and most have been in small numbers. The only previous study to systematically examine these relationships was limited by enrolling only 14 patients, did not use age as a covariate, and was inadequately powered to look at multiple comparisons (14). We found an association between stroke and total Scheltens score as well as with involvement of the internal capsule and pons. This is not surprising, because most symptomatic lacunar infarcts will involve the corticospinal tract in one of these 2 regions. Dementia was related to total Scheltens score but was more strongly related to specific involvement of the SWM. The mechanism of cognitive impairment in CADASIL is uncertain, but it has been suggested that it may relate to disruption of cortical-subcortical or cortical-cortical connections, because of white matter tract damage (17).
Previous psychiatric disorder, which in most cases was depression, was more strongly related to SWM involvement than total Scheltens score. This is consistent with increasing evidence demonstrating an association between white matter lesions and late-onset depression in sporadic patients (18). In contrast to the positive associations between certain regions and stroke, dementia, and psychiatric disease, we found no associations between any regions and either epilepsy or the characteristic reversible encephalopathy that has recently been described in CADASIL (12). We also found no association between involvement of particular regions and migraine; a recent study of 62 CADASIL patients reported reduced occipital white matter involvement in patients without migraine, though the investigators suggested this might represent a chance association due to the multiple comparisons performed (19).
Our study has a number of limitations. First, CADASIL patients were recruited from throughout Great Britain and not all were able to visit our center. In these cases, MR imaging scans were performed locally. Therefore, different acquisition sequences were used. In all cases, however, original scans were reviewed. Second, the scans were reviewed by only one neuroradiologist.
Conclusion
In summary, our data, from the largest MR imaging series in CADASIL, show a highly specific pattern of involvement appearance in most subjects. They highlight the sensitivity of MR imaging in all age groups but demonstrate that involvement is frequently confined to a few regions in younger age groups. Anterior temporal pole involvement, though characteristically confluent when present, may occasionally be absent. Corpus callosum involvement when present may differentiate CADASIL from sporadic small-vessel disease, but it is not severe in most subjects on axial images.
Acknowledgments
We are grateful to Dr. Roswell Martin, for help with clinical data collection and genotyping, and Dr. Yabin Dong, Kelly Gormley, and Dr. Steve Bevan, for assistance with genotyping. CADASIL case identification was assisted by the British Neurologic Surveillance Unit.
Footnotes
This work was supported by the Harrison Lecturership from the Atkinson Morley Neuroscience Research Foundation.
References
- Received December 31, 2004.
- Accepted after revision February 24, 2005.
- Copyright © American Society of Neuroradiology