Abstract
Summary: We report causes leading to and rupture of a carotid ophthalmic aneurysm after treatment by parent vessel occlusion. The aneurysm was initially symptomatic but unruptured.
Carotid occlusion for symptomatic large or giant carotid ophthalmic aneurysm is a well-established treatment (1, 2), which has been widely used for more than 20 years. We report a case of rupture of a carotid ophthalmic aneurysm following parent vessel occlusion.
Case Report
A 49-year-old right-handed woman, with a history of high blood pressure and smoking, came to the emergency department because of recent progressive frontal headache, vomiting, and decreased vision in her left eye. Clinical examination revealed a left superonasal quadranopsia with a left papilledema.
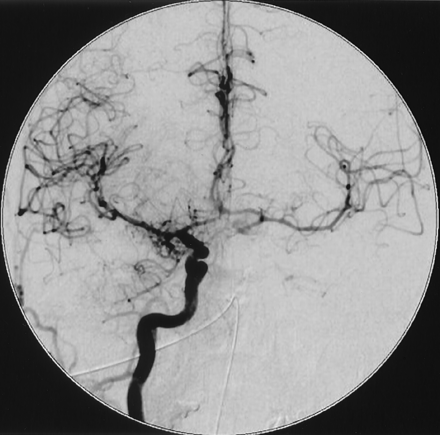
CT findings were negative for blood but showed a left, unthrombosed, giant carotid aneurysm. Angiography confirmed the diagnosis and demonstrated that the aneurysm had a wide neck, was pointing posterosuperiorly, and was arising just distal to the ophthalmic artery (Fig 1 and 2). After initial evaluation, the patient was referred to our department for embolization.
Left carotid angiogram, anteroposterior view, shows a giant carotid ophthalmic aneurysm.
Left carotid angiogram, lateral view, shows that the neck of the aneurysm is just distal to the ophthalmic artery origin.
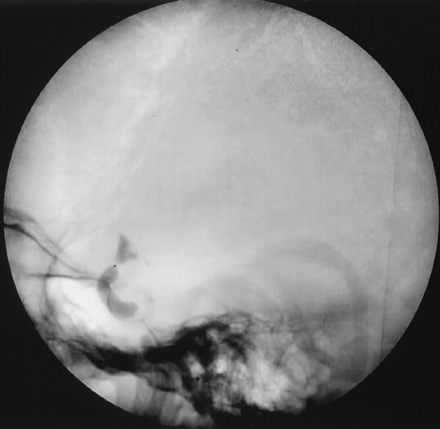
A parent vessel occlusion was scheduled for the following day. With the patient under local anesthesia and systemic heparinization, we performed a test occlusion of the left carotid artery with a detachable gold valve latex balloon (GVB 16, CathNet-Science, Paris, France), inflated at the proximal end of the aneurysm to cover the origin of the ophthalmic artery (Fig 3). This was performed to avoid residual filling of the aneurysm through retrograde flow in the ophthalmic artery. Injection of the right carotid artery showed a good anterior communicating artery filling the entire left carotid territory (Fig 4). A faint retrograde filling of the aneurysm was observed on late phase (Fig 5). Vertebral artery injection revealed no left posterior communicating artery. After a 20-minute occlusion test with controlled blood pressure, no neurologic deficit was noted. The balloon was then detached, and a second identical balloon was detached below the first one for security.
Angiogram, lateral view of the skull, shows inflation of the balloon at the junction between cavernous and carotid ophthalmic segment.
Angiogram5, anteroposterior view, shows Injection of the right carotid artery after occlusion of the left internal carotid artery. Note a good anterior communicating artery supplying the entire left carotid artery territory.
Angiogram5, anteroposterior view, late phase, shows injection of the right carotid artery after occlusion of the left internal carotid artery. Note a faint and delayed retrograde opacification (arrow) of the left carotid ophthalmic aneurysm.
The patient was admitted to the intensive care unit for observation. Neurologic evaluation and blood pressure parameters of the patient were monitored for 72 hours. Special attention was required to keep the systolic arterial pressure above 130 mm Hg. The patient received anticoagulation and corticosteroid therapy for the next 24 hours. The clinical evolution was uneventful, and the patient was released home with acetylsalicylic acid (325 mg po qd) for a week.
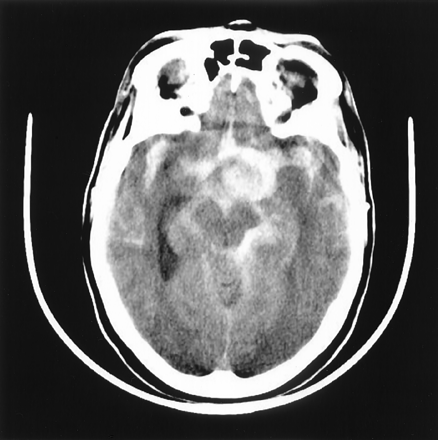
One week after her discharge, the patient was found unconscious at home by her family. On arrival at the hospital, she was in a deep coma. A CT scan (Fig 6) revealed massive subarachnoid hemorrhage. Angiography showed that the left internal carotid artery was still occluded. The two balloons had not moved and had not deflated. There was slow retrograde opacification of the left carotid ophthalmic aneurysm via the anterior communicating artery with stagnation (Fig 7, 8). No other aneurysm or potential source of bleeding was demonstrated.
Nonenhanced CT scan shows an important and diffuse subarachnoid bleeding. Note that the aneurysm is outlined by blood.
Right internal carotid angiogram, anteroposterior view, obtained a few hours after the bleeding, shows opacification of the left internal carotid artery territory, faint retrograde opacification of the left carotid ophthalmic artery aneurysm, and early vasospasm.
Angiogram5, lateral unsubtracted view, obtained after injection of the right internal carotid artery, shows that the balloons did not deflate. Note stagnation of contrast within the aneurysm.
A left frontotemporal craniotomy with a left precoronal ventriculostomy was performed on an emergency basis. The carotid ophthalmic aneurysm was secured by trapping. The patient’s status did not improve with time. The day after, she died of refractory intracranial hypertension.
Discussion
Three to five percent of aneurysms measure 2.5 cm in diameter and are called “giant.” Two thirds are located in the anterior circulation. They come to clinical attention by their propensity to create mass effect and ischemia, but up to one third may present with subarachnoid hemorrhage (3).
The endovascular occlusion of intracranial vessels for aneurysm treatment of unclipable aneurysms is an established procedure (2, 4). This option is considered when the aneurysm is difficult to treat surgically or when the lesion has a high tendency for recurrence after selective endovascular treatment (i.e., large neck, large size). As in this patient, a large or giant carotid ophthalmic aneurysm may be treated by intravascular balloon occlusion of the internal carotid artery (5). The presence of symptoms of mass effect made us decide in favor of parent vessel occlusion.
The site of the occlusion is based on the distribution of flow demonstrated during the test occlusion. When the aneurysm is treated by parent vessel occlusion, the aneurysm is not removed; however, it has been reported that clinical symptoms related to mass effect improved in up to two thirds of cases (3). Because laminar flow is no longer present, thrombosis occurs within the aneurysm. It is thought that the reduction in mass effect results from thrombosis and retraction of the aneurysm wall (1).
If an aneurysm remains in communication with arterial circulating blood, there is still a theoretical risk of rupture, which has been shown with posterior circulation aneurysms. Gurian et al (6) reported a case of rupture of a vertebral aneurysm after parent vessel sacrifice.
Reports of rupture of a giant carotid aneurysm after an internal carotid ligation do not appear often in literature. Matsuda et al (7) and Anson et al (8) described the rupture of a carotid aneurysm after parent vessel occlusion and extracranial-intracranial bypass. To our knowledge, in absence of external carotid–internal carotid2 bypass, rupture of a giant aneurysm after parent vessel occlusion has not been reported for ophthalmic segment aneurysms.
Larson et al (5) have reported 2 cases of delayed subarachnoid hemorrhage in their study of long-term follow-up of 58 patients. In 1 patient, a cerebral angiogram demonstrated a de novo anterior communicating artery aneurysm. In the other patient, a cerebral angiogram demonstrated enlargement and rupture of a previously identified A1-A2 complex aneurysm. In our patient, on the basis of angiographic findings and on surgical observation, we found that there was no de novo aneurysm and that the carotid ophthalmic aneurysm itself had ruptured.
An untrapped aneurysm has a risk of bleeding until it is completely excluded from the arterial flow. An angiogram showing retrograde filling, even faintly, is proof that flow is reaching the aneurysm. To explain why the aneurysm ruptured, we proffer several hypotheses: 1) Even if the flow and arterial pressure seemed faint, the intraaneurysmal flow could have been redirected and thus aimed to a part of the dome where the wall was weak. 2) Enlargement of the aneurysm by acute luminal clot could have led to stretching of the aneurysm wall. 3) Unthrombosed aneurysm has no vasa vasorum thus depending on flow within itself for wall perfusion. Decrease of intraaneurysmal flow would then lead to a decrease in wall perfusion, making the aneurysm susceptible to necrosis and then rupture.
We still consider parent vessel sacrifice to be a valid treatment option, but one must be cautious when filling of the aneurysm, even faintly, persists following occlusion of the parent vessel. On the basis of this case, when some opacification of aneurysm is still demonstrated during the test occlusion, a concomitant selective treatment of the aneurysm with a coil should be considered, just as Gurian et al (6) suggested for posterior circulation aneurysms. However, long-term evaluations and clinical observations must follow to validate this statement because such an attitude may increase the complexity and the complication rate of the procedure.
- Copyright © American Society of Neuroradiology