Abstract
BACKGROUND AND PURPOSE: CSF enhancement on MR images after intravenous administration of gadolinium chelate, which mimics subarachnoid hemorrhage, has been reported. The purpose of this study was to determine whether CSF enhancement can be seen on serial MR images following administration of contrast material in patients with transthyretin-related familial amyloid polyneuropathy (FAP) and to assess other ancillary MR findings.
METHODS: We serially studied T1-weighted and fluid-attenuated inversion recovery (FLAIR) images of the brain before, immediately after, and 3, 6, and 24 hours after contrast administration in 6 patients with genetically confirmed transthyretin-related FAP. By consensus, 2 radiologists assessed the presence, degree, and extent of enhancement of the CSF, leptomeninges, brain parenchyma, and other structures. Statistical analysis was performed to define the difference of the enhancement between the 2 MR imagings.
RESULTS: In 3/6 patients with cysteine-for-tyrosine substitutions at position 114 (Tyr114Cys mutations), marked CSF enhancement was observed on the FLAIR images at 3 and 6 hours and on T1-weighted images at 3 hours after contrast administration. Although there was no significant difference between the 2 MR imagings, leptomeningeal enhancement for these 3 patients was evident only on FLAIR images. The labyrinth and vitreous body was also enhanced on postcontrast delayed MR images of these 3 patients. These enhancements were not observed in the other 3 patients with Val30Met mutation. In none of the 6 patients did images demonstrate parenchymal enhancement of the brain.
CONCLUSION: In FAP patients with Tyr114Cys mutations, contrast material can leak into the CSF. This finding may depend on the subtype of FAP and be more evident with FLAIR images. The enhancement of the leptomeninges, labyrinth, and vitreous body was also seen in the patients.
Transthyretin (TTR)–related familial amyloid polyneuropathy (FAP) is one form of hereditary systemic amyloidosis. It initially presents with polyneuropathy and autonomic dysfunction and progresses to involve many organs (1, 2). It is an autosomal-dominant disorder attributable to mutation in the gene for TTR. More than 100 mutations have been identified as causative gene abnormalities in FAP (3). In the central nervous system (CNS), extensive amyloid involvement of the leptomeninges and subarachnoid vessels in the intracranial and spinal regions has been reported (4, 5). MR imaging findings in the CNS of patients with TTR-related FAP include leptomeningeal enhancement of the spinal cord and brain on contrast-enhanced T1-weighted images (6–8) and thickening of several structures within the spinal subarachnoid space on 3D constructive interference in steady-state images (9). Intracranial hemorrhages have also been observed (6, 8). In our review of MR images of patients with TTR-related FAP, we observed CSF enhancement on fluid-attenuated inversion recovery (FLAIR) and T1-weighted images after contrast administration. Although CSF enhancement after intravenous administration of gadolinium chelate has been reported in various pathologic conditions such as stroke, brain tumors, previous surgery, meningitis, and renal dysfunction (10–16), our review of the literature identified no documentation of CSF enhancement in patients with TTR-related FAP. The CSF enhancement after intravenous administration of gadolinium chelate mimics subarachnoid hemorrhage (10–16), and it is clinically important to know what conditions cause the phenomenon. The purpose of our study was to determine whether TTR-related FAP can cause CSF enhancement on serial studies of contrast-enhanced MR imaging. When the CSF enhancement was seen, we sought to assess other ancillary MR findings.
Methods
Subjects
Included in this prospective study were 6 consecutive patients whose FAP was genetically diagnosed between September 2003 and August 2004 at Kumamoto University Hospital. The patients included 4 women and 2 men, ranging in age from 29 to 52 years (mean age, 39.7 years ± 8.4 [SD]). In cases 1–3, TTR-related FAP was attributable to a substitution of cysteine for tyrosine at position 114 in the TTR molecule (Tyr114Cys mutation). In cases 4–6, there was a substitution of methionine for valine at position 30 (Val30Met mutation). Experienced neurologists (Y.A., T.Y.) recorded their neurologic findings. The 6 patients presented with polyneuropathy and autonomic dysfunction such as diarrhea and/or impotence and other neurologic signs, including hypesthesia, hypalgesia, numbness and dysesthesia of the thigh and lower limbs, and a decrease in the deep tendon reflex. None of the 6 patients manifested renal dysfunction. The patients in cases 1–3 presented with CNS signs and symptoms (ie, transient speech disturbance, hemiparesis, and fluctuating consciousness); patients 2 and 3 underwent liver transplantation 3 and 5 years ago, respectively. In patients 2 and 3, blood TTR levels normalized after liver transplantation. Patient 1 had a cerebral hemorrhage due to systemic amyloid. MR imaging yielded no significant findings in the brain parenchyma of the other five patients. Patient 2 had retinal detachment in the left eye. Patient 3 had undergone vitreous surgery and laser treatments in the right eye because of vitreous opacities due to systemic amyloid. Prior written informed consent to perform contrast-enhanced imaging studies was obtained from all patients or their relatives.
MR Imaging Findings and Interpretation
All MR examinations were performed with a 1.5T MR imaging scanner by using a circularly polarized head coil. Before contrast-enhanced MR imaging studies, axial T1 (TR/TE/excitation, 670/14/1), T2 (TR/TEeff/excitation, 3500/96/2; echo train length, 7), and FLAIR (TR/TEeff/inversion time, 6000/120/2000; echo train length, 17) images were obtained. The T1- and T2-weighted and FLAIR sequences were acquired at a section thickness of 5 mm, a 256 × 256–512 matrix, and a 22-cm field of view.
Serial T1-weighted and FLAIR images of the brain were performed immediately after and 3, 6, and 24 hours following the intravenous administration of gadolinium diethylene triamine pentaacetic acid (DTPA) (0.1 mmol/kg). The images were transferred to a workstation and reviewed in one session by 2 experienced neuroradiologists (M.Y., T.H.). Each viewer independently evaluated pre- and postcontrast FLAIR and T1-weighted images presented side by side at the same time. In cases of disagreement, final judgment was based on the consensus of the 2 reviewers. They rated the images for the degree of contrast enhancement of the leptomeninges, CSF, brain parenchyma, labyrinths, and eyes. The degree of enhancement was compared with precontrast MR images and graded as marked (++), mild (+), equivocal (±), and none (−). In addition, the extent of contrast enhancement was recorded.
Statistical Analysis
The Wilcoxon signed-ranks test was used to define the difference of meningeal and CSF enhancement at each interval between postcontrast FLAIR and T1-weighted images. P values <.05 were considered significant.
Results
Leptomeningeal and Parenchymal Enhancement of the Brain
As shown in the (Table), patients 1–3 manifested leptomeningeal enhancement. On T1-weighted images obtained immediately after contrast administration, this enhancement could not be differentiated from the enhanced cortical vessels (Fig 1A), and it was therefore recorded as equivocal. By contrast, FLAIR images revealed marked leptomeningeal enhancement, especially in the superior cerebellar cisterns, Sylvian fissures, interhemispheric fissures, and cerebral sulci. In statistical analysis, there was no significant difference for abnormal leptomeningeal enhancement at each imaging timing between the 2 MR imagings.
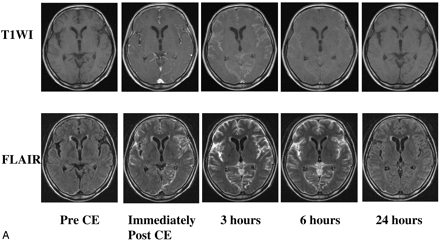
Case 3, a 52-year-old woman with Tyr114Cys-type TTR-related FAP. Pre- and postcontrast serial MR images at the level of the basal ganglia (A) and the cerebellum (B) are shown. T1-weighted and FLAIR images are presented in the upper and lower rows, respectively.
A, On T1-weighted MR images obtained immediately after contrast administration, leptomeningeal enhancement is equivocal because it is similar to enhanced cortical vessels. Marked enhancement of the leptomeninges is obvious on FLAIR images obtained at the same time points. At 3 hours postcontrast, there is marked CSF enhancement on both sequences. The contrast between the CSF and the brain parenchyma appears greater on FLAIR than T1-weighted images. At 6 hours postcontrast, the FLAIR image shows marked—and the T1-weighted image mild—CSF enhancement. CSF enhancement has almost disappeared on the 24-hour T1-weighted image. Mild CSF enhancement persists in some sulci on the 24-hour FLAIR image; some motion artifacts are present. There is no apparent enhancement of the intraventricular CSF or the brain parenchyma on either sequence at any time point examined.
B, On the precontrast FLAIR image, slightly hyperintense areas are seen in the right vitreous body (arrow). On images obtained immediately after contrast administration, there is mild enhancement of the leptomeninges of the left cerebellar hemisphere (arrowhead) and right auditory canal (arrow). There is no apparent leptomeningeal enhancement on T1-weighted images. On 3-hour FLAIR images, there is marked CSF enhancement in the prepontine and cerebellopontine angle cisterns and auditory canal (arrowhead). Mild enhancement of the right labyrinth (arrow) and bilateral vitreous bodies is observed. On the 3-hour, T1-weighted image, there is mild enhancement only in the prepontine and cerebellopontine angle cisterns. The 6-hour FLAIR image shows marked CSF enhancement in the prepontine and cerebellopontine angle cisterns and marked enhancement of the bilateral labyrinths (arrows). Marked and mild enhancement are seen in the right and left vitreous on the FLAIR images. The 6-hour, T1-weighted image demonstrates mild enhancement in the prepontine and cerebellopontine angle cisterns and auditory canals; there is equivocal enhancement in the right labyrinth (arrow). On 24-hour images, there is almost no CSF enhancement on both sequences. Mild enhancement is seen in both labyrinths (arrows) on both sequences. On the FLAIR image, marked and mild enhancement persist in the right and left vitreous bodies. On T1-weighted images obtained 24 hours after contrast administration, there is no obvious enhancement of the vitreous.
Summary of serial changes in leptomeningeal and CSF enhancement on postcontrast MR images
We observed no abnormal enhancement in the brain parenchyma in any of the 6 patients at any time on T1-weighted or FLAIR images.
Enhancement of CSF on Serial Contrast-Enhanced MR Imaging
In cases 1–3, there was marked and diffuse CSF enhancement on FLAIR images obtained 3 and 6 hours after contrast administration and on T1-weighted images obtained 3 hours after contrast administration (Fig 1A; Table). Enhancement was seen primarily in the prepontine, cerebellopontine angle, basal, and superior cerebellar cisterns and in the Sylvian and interhemispheric fissures and cerebral sulci. On 24-hour FLAIR images, there was mild CSF enhancement in the cerebral sulci (Fig 1A). The intraventricular CSF was slightly enhanced on 3- and 6-hour FLAIR images in one patient (case 2), though the degree of enhancement of the intraventricular CSF was much smaller than that of the subarachnoid-space CSF. No such finding was made in any of the other 5 patients. There was no statistically significant difference for abnormal leptomeningeal enhancement at each interval between the 2 MR imagings. We observed no abnormality in the choroid plexus in any of the 6 patients at any time on T1-weighted or FLAIR images.
Abnormal Enhancement of Other Structures
In cases 1–3, FLAIR images revealed marked enhancement in the labyrinth at 6 hours; it was mild on images obtained at 24-hour postcontrast administration (Fig 1B). In the other 3 cases, there was no clear enhancement of the labyrinth on any of the images.
In the same 3 patients, the vitreous body of the bilateral orbits enhanced on FLAIR images; marked enhancement was seen on 6- and 24-hour FLAIR images (Fig 1B). On T1-weighted images, only case 2 demonstrated enhancement of the vitreous body at 6 and 24 hours. None of the other 3 patients manifested vitreous body enhancement at any phase on any images.
Discussion
CSF enhancement after intravenous administration of gadolinium chelate in a number of disorders may mimic subarachnoid hemorrhage (10–16). To avoid unnecessary examinations, such as lumbar puncture, it is clinically important to know what conditions cause this phenomenon. CSF enhancement after the intravenous administration of gadolinium chelate has been reported in various pathologic conditions (eg, stroke, meningitis, intra- and extraaxial brain tumors, previous surgery, and renal dysfunction) [10–16]). This is thought to be due to leakage of the contrast material into the subarachnoid space due to blood-brain barrier (BBB) breakdown, neovascularization, or a high serum concentration of contrast medium (14, 16).
TTR, also known as prealbumin, is a 127-amino acid protein. It is a tetrameric human plasma protein that transports thyroxine and retinol and is synthesized in the liver, choroid plexus, and retinal pigment epithelium. TTR-related FAP is an autosomal-dominant disorder attributable to mutation in the gene for TTR. It initially presents with polyneuropathy and autonomic dysfunction and progresses to involve many organs (1, 2). More than 100 mutations have been identified as causative gene abnormalities in FAP (3). Because of this variety in TTR gene mutations, the clinical phenotypes of TTR-FAP differ significantly. Mutant TTRs are predominantly synthesized in the liver and deposited as extracellular twisted β-pleated sheet fibrils in peripheral somatic and autonomic nerves and other organs. In the CNS, extensive amyloid thickening of the leptomeninges and subarachnoid vessels in the intracranial and spinal regions has been reported (4, 5). Liver transplantation is thought to hold promise for saving the lives of patients with TTR-related FAP (17, 18).
We observed leptomeningeal enhancement in 3 of our 6 patients. Although statistical significant differences were not obtained, it was more clearly evident on postcontrast FLAIR than T1-weighted images. On the latter, it was not possible to differentiate leptomeningeal enhancement from enhanced cortical vessels. Because of suppression of CSF signal intensity, contrast-enhanced FLAIR images are valuable for the detection of sulcal or leptomeningeal lesions (19–22). Unlike postcontrast T1-weighted images, on postcontrast FLAIR images, slow-flowing blood is not usually hyperintense. This may partly account for the clear difference between enhanced meninges and enhanced cortical vessels on FLAIR images (20) and renders FLAIR imaging more valuable than T1-weighted imaging in these cases.
Postmortem studies of patients with classic TTR-related FAP often disclosed extensive amyloid thickening of the leptomeninges and subarachnoid vessels in the intracranial and spinal regions (4, 5). The mechanism of meningeal enhancement is not fully understood. We posit that contrast enhancement of pial and arachnoid membranes may be due to an effect of amyloid deposits on the meninges and vessel walls, to extracellular leakage of contrast material through an impaired blood-cord, BBB, or blood-CSF barrier, to insufficient washing out of contrast material due to altered vascularity, or to vascular stasis within abnormal leptomeningeal vessels or tissues (7).
Among our 6 patients, one (case 1) had a cerebral hemorrhage; the other patients manifested no significant intraparenchymal abnormalities on MR imaging. Although earlier histopathologic studies had demonstrated some amyloid deposits in the wall of cerebral vessels (23, 24), we observed no abnormal parenchymal enhancement in stationary or serial studies. We postulate that a BBB breakdown is rare in the brain parenchymal region.
We found CSF enhancement in 3 patients with leptomeningeal enhancement. Autopsy studies of patients with TTR-related FAP reported no neovascularization of leptomeningeal vessels or piaarachnoid membranes (4, 5). None of our 6 patients manifested renal dysfunction, and a standard dose of contrast medium was administered to all patients. Previous and current findings suggest that the association of subarachnoid leptomeningeal vessels with amyloid deposits may result in a blood–CSF barrier breakdown, allowing the transition of contrast material from leptomeningeal vessels into the subarachnoid space.
Leakage of contrast medium into the CSF shortens T1 relaxation in the CSF. Because the inversion pulse in FLAIR sequences introduces considerable T1 weighting and affects T1 relaxation time (25), leakage of contrast into the CSF introduces high CSF signal intensity on FLAIR images. Phantom and animal studies disclosed the extreme sensitivity of FLAIR imaging to changes in T1 relaxation of the CSF (14), and changes in the appearance of the CSF were apparent even at gadolinium concentrations as low as 0.007 mmol/L (14). In healthy humans, however, this effect was negligible when FLAIR images were obtained immediately and 110 minutes after the administration of a standard dose of gadolinium-DTPA (0.1 mmol/kg) (16). In our study, the 3 patients without leptomeningeal enhancement (cases 4–6) did not manifest CSF enhancement on serial contrast- enhanced MR imaging.
Although there was no apparent ventricular CSF enhancement in 2/3 patients with the subarachnoid-space CSF enhancement, one (case 2) demonstrated slight enhancement of intraventricular CSF on FLAIR images obtained 3 and 6 hours after contrast administration. In this case, the degree of enhancement of the intraventricular CSF was much smaller than that of the subarachnoid-space CSF. We then observed no abnormality in the choroid plexus in all patients on MR images. Therefore, we postulate that enhancement of the intraventricular CSF may represent reflux from the subarachnoid CSF and that the observed leakage of contrast medium derives from subarachnoid vessels.
We found labyrinth enhancement in patients with CSF enhancement; it was strongest 6 hours after contrast administration. The subarachnoid space around the brain is continuous with the perilymphatic space of the bony labyrinth via a tiny canal in the temporal bone. Because the time of peak enhancement was later in the labyrinth than the CSF, we speculate that CSF-containing contrast medium transferred to the labyrinth.
The differential diagnosis of vitreous enhancement includes renal failure, various forms of retinopathy, retinitis, and retinal laser treatments (26, 27). This enhancement, however, has not been reported in patients with TTR-related FAP. In our study, the vitreous enhancement was seen in 3 patients with Tyr114Cys mutation. Although 2 of the 3 patients had previous disturbance of the unilateral eye, this MR finding was observed even in the eye without the disturbance. Therefore, the vitreous enhancement is considered to be associated with systemic amyloid. Amyloid deposits are commonly found in the eyes of FAP patients with some specific TTR mutations (8, 28, 29). These deposits have been found in the retina, vitreous body, choroids, cilliary body, perivascular area in and around the nerves, and stroma of the extrabulbar tissue (29). When both eye and meningeal lesions are seen in FAP patients, this is known as oculoleptomeningeal amyloidosis (30). A factor contributing to the amyloid deposits is the production of TTR by the retina (31). Vitreous enhancement may be associated with the disruption of the blood-retinal barrier. Further investigations are needed to clarify the mechanism underlying vitreous enhancement.
There are some limitations to our study. First, our prospective study included only 6 FAP patients with 2 types of TTR mutation. FAP with the Val30Met mutation can lead to oculomeningeal lesions and meningeal enhancement on contrast-enhanced MR imaging (8); in our series, abnormal enhancement was found only in patients with the Tyr114Cys mutation. Among our patients, those with the Val30Met mutation were relatively younger and free of CNS signs and symptoms. It is possible that older patients would present with more definite MR findings as amyloid deposits accumulate with age. Further studies with larger populations are required to determine whether the abnormal MR findings are associated with CNS signs and symptoms or with patient age. Second, 2/6 patients we studied had received liver transplants. Although transplantation improved blood TTR levels, these cases had persistent abnormal enhancement. The reason for the persistent abnormal enhancement is not known. The effect of amyloid deposition to the leptomeninges may persist even if blood TTR levels are normalized. Further MR imaging studies are necessary to determine the effect of liver transplants on the CNS in patients with TTR-related FAP. Third, statistically significant differences were not obtained between the 2 MR imaging methods. This is probably due to the small sample size of our study. Further studies with large sample size are needed to clarify the difference.
Conclusion
In patients with TTR-related FAP with Tyr114Cys mutations, we observed the leakage of contrast medium into the CSF. This leakage of contrast medium was not observed in patients with Val30Met mutation. This phenomenon may depend on the subtype of FAP. Because this phenomenon mimics subarachnoid hemorrhage, it is clinically important to know it to avoid unnecessary examinations. We postulate that the leaked medium derived from leptomeningeal vessels. On serial contrast-enhanced FLAIR images, we noted the abnormal enhancement of the labyrinth and vitreous body. In the evaluation of abnormal enhancements, FLAIR images tended to demonstrate enhancement more definitely than did T1-weighted images. We recommend that postcontrast serial FLAIR images should be obtained to evaluate patients with TTR-related FAP.
References
- Received November 5, 2004.
- Accepted after revision February 16, 2005.
- Copyright © American Society of Neuroradiology