Abstract
BACKGROUND AND PURPOSE: Limited data exist on the natural history of thoracic kyphosis in elderly patients. The purpose of this study was to determine the statistical distribution of the thoracic kyphotic angle (TKA) measurement in older patients without vertebral body abnormalities when compared with a young population.
METHODS: The TKA was measured by Cobb angle on digital lateral chest radiographs in 90 patients >65 years of age, 60 patients 51–65 years of age, 67 patients 36–50 years of age, and 63 patients 18–35 years of age. Patients with vertebral compression, vertebral body angulation, congenital anomaly, or significant scoliosis were excluded.
RESULTS: In patients >65 years of age, average TKA was 41.9°, but the distribution was unexpectedly bimodal, with a low mode at 28.3° and an upper mode at 51.5° (P < .001). Elderly women and men independently demonstrated a bimodal TKA distribution. Two-thirds of elderly women and half of elderly men had a TKA >40° (upper mode). In young patients, average TKA was 26.8°. In middle-aged patients, TKA was intermediate and nonbimodal.
CONCLUSION: The TKA distribution in elderly patients (>65 years) without vertebral body abnormality is unexpectedly bimodal (non-normal distribution) with a subpopulation of patients significantly more affected by extreme kyphosis. Extreme thoracic kyphosis therefore occurs independently in a large subset of people, in the absence of vertebral wedge compression. The development of extreme thoracic kyphosis might contribute to excess biomechanical stress in the spine and may identify a population at risk for future vertebral compression fracture in particular at the thoracolumbar junction.
The primary complication in osteoporosis is fracture, resulting in a significant increase in morbidity and mortality (1–4). The frequency of vertebral body compression fracture is estimated to occur in ∼15% of osteoporotic white women and 5% of osteoporotic white men, leading to vertebral body height loss or angulation and the development of progressive thoracic kyphosis (1, 5). Expectant management of painful vertebral collapse is commonly attempted, but advanced treatments are also available including vertebroplasty and kyphoplasty (5–9). Treatments are designed to stabilize the actively compressing vertebrae and treat the pain that accompanies collapse. Issues that affect proper choice among treatment procedures include patient clinical condition and the potential of improvement in vertebral height or angulation leading to better medical stability (2, 10, 11).
Limited data exist on the natural history of thoracic kyphosis in older patients. There is the suggestion that the normal thoracic kyphotic angle (TKA) progressively increases with age, but studies documenting the age characteristics of the TKA in the thoracic spine are few (12–16). A study of the TKA measured by Cobb angle on lateral thoracic spine images suggests increasing angle with age and an increased angle in the elderly, but few patients were included in the older population (13).
The purpose of this study was to determine the statistical distribution of the TKA measurement in older patients without vertebral body compression when compared with a young population.
Methods
Institutional review board approval was obtained for this retrospective study. Sixty-three patients 18–35 years of age (average age, 24 years; 27 men and 36 women), 67 patients 36–50 years of age (average age, 44 years; 33 men and 34 women), 60 patients 51–65 years of age (average age, 58 years; 30 men and 30 women), and 90 patients >65 years of age (average age, 74 years; 42 men and 48 women) were sequentially identified from 2 affiliated university hospitals that had routine inpatient and outpatient standard erect posterior-anterior (PA) and lateral chest radiographs, but no vertebral body shape abnormality. Studies were obtained by using standard direct radiography (DR) or computed radiology (CR) filming technique with focal spot to film distance 72 inch, 14 × 17 CR cassette or direct capture, central ray midthoracic vertebrae central chest (17). CR or DR images were evaluated by using a picture archiving and communications system (PACS) image review network and workstation.
Current digital radiographic imaging by using DR or CR technology and PACS computer workstation display allows sufficient image dynamic range or “latitude” to clearly identify upper and lower thoracic vertebrae and endplates on most standard lateral chest images. Therefore, unlike earlier studies, we were capable of obtaining measurements throughout the entire thoracic spine. In addition, computer-directed angle measurements allow for highly accurate Cobb angle determination as opposed to use of a typical plastic goniometer.
Only patients with acceptable erect PA and lateral examinations and normal-appearing spines, as judged by visual inspection by an experienced neuroradiologist, were included in this study. Exclusion criteria included poor vertebral visualization limiting the ability to measure spine curvature, any objective visual evidence of vertebral body compression (loss of vertebral body height relative to the other thoracic vertebrae or vertebral body wedge shape or angulation), or significant scoliosis.
The intrinsic thoracic kyphosis is related to the normal morphometry of the thoracic vertebrae and disk spaces. Thoracic vertebrae typically demonstrate slightly asymmetric shape with reduced anterior vertebral height relative to the posterior vertebral dimension varying from 5% to 15% (18–21). Methods using quantified vertebral dimensions and thresholds beyond the normal 5%–15% asymmetry in anterior to posterior dimensions have been employed to diagnose vertebral fracture (20), but visual inspection by experienced radiologists continues to be the standard of fracture determination (21). For this reason, the simpler approach of visual inspection for vertebral compression and patient exclusion was considered valid and correct.
Exclusion criteria were categorized into 4 distinct groups: (1) isolated slight wedge deformity; (2) single or multiple significant wedge compressions; (3) isolated vertebral compression with loss of height but no wedge shape; and (4) poor spine visualization precluding accurate vertebral body assessment (because of superimposed lung disease, significant osteopenia, scoliosis, or technical factors such as penetration or spine cutoff). These criteria are summarized in Table 1. Case rejection due to poor spine visualization was consistent among the 4 groups (range, 7.5%–10.3%) and related to superimposed lung disease, osteopenia, or scoliosis. Isolated slight wedge deformity was judged as a distinct visible vertebral body wedge shape out of character or proportion to the shape of the other vertebral bodies in that individual patient in whom old or new vertebral compression fracture could not be confidently excluded. This was a frequent cause for exclusion in the younger patient group (23.1%) but was a consistently low cause of exclusion in the other age range groups (4%–6.3%). Single or multiple wedge compression increased among the 4 groups, from a low of 22.1% in young patients to 67.9% in the elderly population.
Inclusion and exclusion groups
Cobb angle measurements of the thoracic spine were obtained on the lateral chest examination by using the traditional previously defined technique employing perpendicular lines drawn to the endplates of T1 or T2 and T12 by using the postprocessing software tools available on the workstation and tabulated (Fig 1A, -B). Two observers agreed upon levels considered acceptable for reliable measurement. Cobb angle measurements were made independently, and final measurements were agreed upon by consensus between the observers. Differences between the age groups along with differences between men and women were separately tabulated, evaluated, and compared.
Workstation measurement of thoracic spine Cobb angle.
A, Initial angles placed perpendicular to the upper vertebral endplate of T2 (black arrow) and lower vertebral endplate of T12 (white arrow) with the angle measurement function available on the Stentor workstation system.
B, Cobb angle measurement obtained by angle measurement of the intersection between perpendiculars to the vertebral endplates (black arrowhead).
Statistical assessment was accomplished using the SAS software package, PROCAPABILITY statistical analysis software function, SAS software release 8.2 (SAS Institute, Cary, NC). Assessment of a bimodal distribution is unique and involves analysis of the probability of exclusion of fit to a normal distribution. Adherence or nonadherence to normal distribution was computed by using goodness-of-fit tests and χ2 tests of known cell probabilities (22). Choice of cell size for column designation is intrinsically determined by sample size being evaluated (22). Statistical significance was considered present when P < .05.
Results
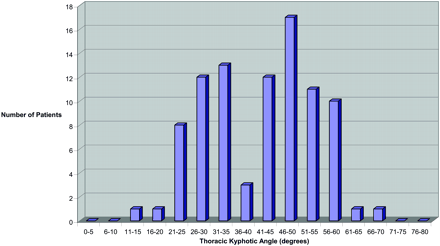
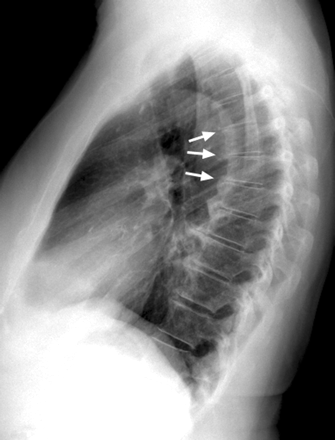
The results are summarized in Table 2 and Figs 2–4. A typical example of exaggerated TKA without vertebral compression in an elderly man is present in Fig 5. The average TKA varied from 26.8° to 42.0° (Table 2). These values compared favorably with the previous reported results of Fon et al (13).
TKA for men and women >65 years of age.
Average thoracic kyphotic angle (TKA)
In patients >65 years of age (Fig 3), the distribution of the TKA was distinctly bimodal (non-normal distribution; P = .001) with a lower mode of 28.3 ± 5.2° and a second upper mode of 51.5 ± 6.3°. Separation between modes appeared to be at a TKA of 40°. In patients 18–35 years of age, 36–50 years of age, and 51–65 years of age, the distributions were more symmetric and χ2 goodness of fit did not exclude a normal distribution.
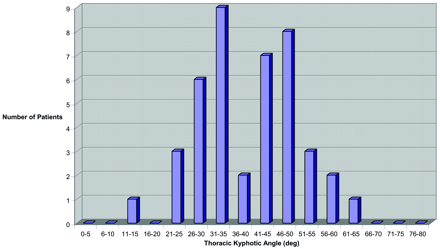
TKA for men >65 years of age.
Both older men and women (>65 years of age) independently demonstrated a bimodal distribution (Figs 3 and 4). In older women, the average TKA was 43.6°, with a bimodal distribution (non-normal distribution P = .01), demonstrating an upper mode of 51.6 ± 6.4° and lower mode of 27.8 ± 5.3°. In older men, the average TKA was 40.0°, with a bimodal distribution (non-normal distribution; P = .02), demonstrating an upper mode of 51.4 ± 6.3° and a lower mode of 28.6 ± 5.2°. The lower mode TKA of both older men and women was interestingly similar to the average TKA for younger patients (26.8°) in our study population as well as the younger patients previously reported by Fon et al (13). A significant fraction of older men (21/42 [50%]) and women (31/48 [65%]) were in the upper mode of the bimodal distribution, having an extreme TKA (>40°).
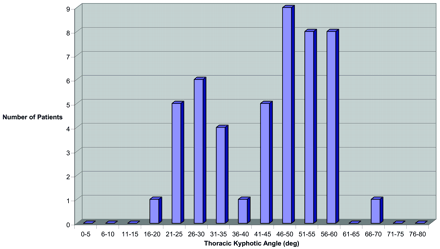
TKA for women >65 years of age.
The distribution of the TKA in young women (18–35 years of age) was also bimodal (non-normal distribution; P = .04), similar to the distribution encountered in older patients. The average TKA in young women was 28.4°, similar to young men, but with a lower mode at 16.1 ± 4.6° and an upper mode at 37.2 ± 5.6°.
The age distribution of patients >65 years is presented by histogram in Figs 6 and 7. The distribution in older men demonstrates progressively fewer subjects with advancing age (Fig 6; average age, 74.8 years), possibly reflecting the age characteristics of the male population in our region. This age decline was seen in both lower mode kyphotic angle (<40°; average age, 73.7 years) and extreme thoracic curvature (>40°; average age, 76.5 years) subsets.
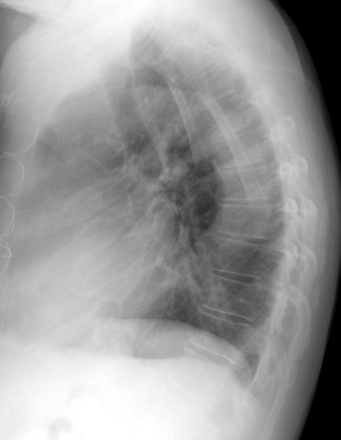
Lateral chest radiograph of a 70-year-old man with an extreme thoracic kyphotic angle (65° Cobb angle) but no vertebral body compression or angulation.
Age distribution for men >65 years of age.
Age distribution for women >65 years of age.
In older women, a more uniform age distribution was present with a greater number of patients >80 years of age (Fig 7; average age, 75.4 years). In older women with lesser thoracic curvature, a broad representation of low and high ages were present, including several women 86–90 years of age (average age, 75.9 years). In older women with extreme thoracic curvature, there was slightly greater number of patients in the lower end of the age range, with an average age of 75.2 years.
Therefore, the presence of the 2 modes in older men and women did not appear to be an age-related feature of the patient population or reflect age selection bias.
Discussion
It is generally accepted that the thoracic kyphotic angle increases with advancing age, in particular in older women. The “dowager hump” is well recognized, and most people equate this with underlying vertebral compression deformity or angulation (2, 14). Increasing thoracic kyphosis, in particular when linked to back pain, is considered a signature of possible vertebral body compression fracture and usually prompts spine imaging. Finding vertebral body compression is not unexpected, and reports of “no fracture” are simply taken as positive news for the patient.
Studies demonstrating an increase in thoracic kyphosis with age are available, but only a limited number of measurements of TKA have been published in patients >60 years of age or in patients without vertebral compression (12, 13). Our data for the average TKA in young, middle-aged, and older patients alike is in excellent agreement with previously published results in normal patients without compression, which suggests they are intrinsically correct (13). It is the finding of a bimodal distribution in older men and women (as well as younger women) that is unexpected and surprising.
The finding of a bimodal distribution fundamentally suggests that 2 populations are actually present in elderly men and women without vertebral compression: (1) those who tend to develop excessive curvature and (2) those who remain near the baseline or younger thoracic curve. Our patient population may represent those who have longevity but retained sufficient bone mineral to resist vertebral compression while engaging in their normal life activities. Sustained vertebral strength would allow any natural tendency to develop thoracic curvature from factors other than vertebral compression to be expressed and visualized. Our study may be observing a unique subset of the elderly population.
A group of clinical factors could contribute separately or in combination to the development of an exaggerated thoracic curve without fracture. In the absence of vertebral compression fracture, changes in the spine support tissues (ie, ligaments, tendons, disk annulus and nucleus) or supporting musculature could lead to a progressive increase in curvature, as has been suggested by other authors. In a longitudinal study of thoracic kyphosis conducted by Milne and Williamson (12), most progressive increase in thoracic curvature in this population could be explained by vertebral body changes (ie, compression or wedge deformity). A component of the developing curvature, however, was clearly due to changes in the soft tissues, presumably from “age-related effects” such as reduced muscle tone or changes in the intervertebral discs (12).
A primary factor that likely contributes to the exaggerated thoracic curve is asymmetric disk degeneration (12, 14–16, 23). Degenerative disk changes were certainly common in our older patient population and contributed to kyphosis in many individuals (Fig 8). Asymmetric loss of anterior disk height with kyphosis has been observed in studies containing a mixture of vertebral compressions and anterior wedge deformity, in addition to degenerative disk changes (15, 16). Support contributed by the costovertebral junctions may limit facet degenerative changes, leading to anterior more than posterior loss of disk height with thoracic degenerative disk disease.
Lateral chest radiograph of a 77-year-old woman with degenerative disk disease and asymmetric disk height loss (white arrows) contributing to increased thoracic kyphotic angle (56° Cobb angle).
A second possible cause of increasing thoracic curvature is reduced thoracic extensor muscular tone (24). Spinal extensor muscle strength diminishes with age and extensor muscle strength has been shown to be reduced in osteoporotic women (25, 26). In addition, a correlation between the measured thoracic kyphosis and decreasing muscular strength has been demonstrated (27).
Natural hypermobility represents a third potential cause of exaggerated thoracic kyphosis (28, 29). General hypermobility with multijoint hyperflexibility is present in 10%–15% of the population (labeled benign joint hypermobility syndrome), and single joint hypermobility has been documented in as much as 27% of the population (28–31). A familial relationship is common, and this benign hyperflexibility may represent a variant of Ehler-Danlos syndrome (Ehler-Danlos III; 29). Hypermobility is more common in women (women : men, 2 : 1 single joint expression, 3 : 3–8 : 1 with multijoint expression) and frequently involves the spine (40% with single joint expression, 90% or higher when 4 or more joints are involved), and a tendency to osteopenia is reported (28, 32). The finding of a bimodal distribution of the TKA in young women could represent the spine expression of natural hypermobility and flexibility.
A fourth factor that could contribute to increasing thoracic kyphosis is postmenopausal hormonal changes with weakening of spine ligament support (including the annulus). Postmenopausal estrogen decline has been implicated in altered tissue support and muscular tone with loss of pelvic floor support (incontinence) and facial tone (wrinkles) in elderly women (33, 34). Collagen loss and altered connective tissue integrity have been implicated in these disorders as a cause of weakening tissue strength (35). Of interest, the incidence of joint hypermobility is increased in women with genitourinary prolapse (36, 37). Skin collagen has been reported as decreased in the postmenopausal state, and reversal can be demonstrated with estrogen therapy (38, 39).
These 4 factors (asymmetric disk degeneration, weakened muscular tone, intrinsic hypermobility, and endocrine-related collagen weakening) may be contributing to kyphosis in the elderly in the absence of vertebral compression, alone or in combination. Patients in the lower mode may simply represent those with less spine flexibility or greater stiffness with a retained younger curve shape. More important, the bimodal distribution in the elderly without vertebral compression suggests that the average kyphotic angle measured in elderly cohorts underestimates the subset with more extreme hypercurvature and potential biomechanical risk.
Biomechanical forces have long been considered a potential factor leading to vertebral compression in osteoporosis (40–43). Studies have demonstrated substantial increase in vertebral body stress with potential for compression during routine daily activities. Carrying a moderate weight (bag of groceries) or the act of lifting while bending foreword (lifting a bag from the car or opening a heavy window) can result in vertebral body forces that meet or exceed failure strength in the presence of osteoporotic bone (40–43). Chronic injury with the development of fatigue fracture is also recognized (40). Although many osteoporotic vertebral compression fractures can be related to an event such as a fall or heavy lifting, a substantial number of vertebral fractures develop without a clear cause (44, 45).
A naturally developing extreme TKA in the elderly could be an important contributing factor in the development of vertebral body compression fractures in osteoporosis. Increased TKA would lead to exaggerated baseline biomechanical stress on the lower thoracic vertebrae, thoracolumbar junction, and lumbar spine. Additional biomechanical stress related to activity (such as foreword bending) would further increase baseline forces that might exceed osteoporotic vertebral body strength. This effect could occur independently or in combination with acquired vertebral wedge compressions. In addition, the constant presence of increased baseline vertebral compressive force could lead to “repetitive” vertebral body injury with development of a “fatigue-like” fracture (40, 43). The most common osteporotic vertebral fractures occur at T8, T12, L1, and L4, and the thoracic kyphosis is known to produce its greatest axial load effects at T8 (5, 44, 45). Such an effect has been suggested in one study in which the incidence of thoracic vertebral body wedge deformity and fracture was increased in osteoporotic women with excessive thoracic kyphosis by using an upper limit of normal as 50° for the thoracic curve (14). In this study, the incidence of exaggerated curve was 80% when 4 or more vertebral compressions were present and 40% when 1–3 compressions were present. Exaggerated kyphosis was also noted in 19% of women without vertebral compression (14).
In our study, a larger percentage of elderly women (65%) had a TKA in the upper mode as compared with elderly men (50%). This difference could contribute to the greater frequency of osteoporotic vertebral compression in women than in men (1, 2). The cause of the differences in TKA distribution between older men and women is not clear. The age distribution in elderly men demonstrated a moderate progressive decline in both low and extreme thoracic curvature groups (Fig 6). Elderly women demonstrated a more even age distribution with a decline in representation beyond 80 years of age (Fig 7). This parallels the age demographics of our region. The greater number of women with extreme curvature could simply reflect the greater longevity of women compared with men and therefore age representation of women in the older population. This may account, at least in part, for the greater proportion of women with extreme curvature in our study. Other factors that may contribute to extreme kyphosis (hypermobility, endocrine collagen changes, and muscular strength) could affect women to a greater degree than men.
An exaggerated TKA in the elderly may be an important factor to consider when choosing between conservative management, vertebroplasty, and kyphoplasty as treatment approaches for painful vertebral fractures (5–9, 46). Painful vertebral compression fractures frequently demonstrate mobility when stressed by extension, and improved vertebral height with vertebroplasty is common (47). The value of restoring vertebral body height by vertebroplasty or kyphoplasty may be important, but its role needs to be considered in the context of the normal progression of the thoracic curve.
Conclusion
The thoracic kyphotic angle in the elderly patient without vertebral compression demonstrates an unexpected bimodal distribution defining a subpopulation significantly more affected by extreme kyphosis. This suggests that the average thoracic kyphotic angles previously reported in the elderly without vertebral compression may underestimate the fraction of people that develop excessive thoracic curvature. Extreme thoracic kyphosis in the elderly, independently or in combination with vertebral wedge compression, might identify a population at increased risk for vertebral compression fracture due to biomechanical stress. In addition, the relative impact of vertebral body height restoration or improvement in kyphotic angle must be considered in light of this data when treating vertebral compression fractures with the vertebroplasty or kyphoplasty techniques.
Footnotes
Presented at the 7th symposium of the American Society of Spine Radiology in Miami Beach, FL, February 15–19, 2004, and at the 42nd annual meeting of the American Society of Neuroradiology in Seattle, WA, June 1–7, 2004.
References
- Received November 15, 2004.
- Accepted after revision February 25, 2005.
- Copyright © American Society of Neuroradiology