Abstract
Summary: Wernicke encephalopathy is a neurologic disorder that results from thiamine deficiency. It is associated with a classic triad of symptoms consisting of ataxia, ocular motor cranial neuropathies, and changes in consciousness. We report 3 cases of Wernicke encephalopathy in which MR imaging, including diffusion-weighted imaging, was performed at the onset and during follow-up. MR imaging findings were correlated with the clinical status of both the acute and chronic stage of Wernicke encephalopathy.
Wernicke encephalopathy is a neurologic disorder that results from thiamine deficiency. It is associated with a classic triad of symptoms consisting of ataxia, ocular motor cranial neuropathies, and changes in consciousness (1, 2). The regions of the brain that have been found to be pathologically involved in Wernicke encephalopathy include the mamillary bodies, periaqueductal regions, medial thalami, third ventricular walls, pons, medulla, basal ganglia, and even the cortical gray matter (3). Many studies have clarified the MR imaging features of acute Wernicke encephalopathy. The short-term follow-up study by MR imaging has also been documented previously. The present case report attempts to correlate MR imaging findings, including those of diffusion-weighted imaging, with the clinical status of both the acute and chronic stages of Wernicke encephalopathy.
Case Reports
Case 1
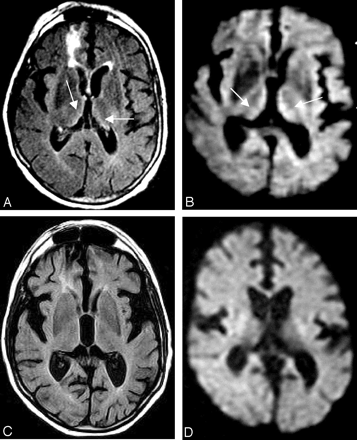
A 23-year-old woman was brought into the emergency department with 2 days of lower extremity weakness and obtundation. She had a 4- to 5-month history of dysphagia, nausea, and vomiting and documented 25-kg weight loss. Approximately 1 month before her examination, she underwent a Heller myotomy for treatment of achalasia. However, this surgical procedure did not provide the anticipated relief. The patient continued to have severe nausea with occasional vomiting and had decreased oral intake during the few days before the examination. A neurologic examination revealed marked horizontal and vertical gaze nystagmus and bilateral sixth nerve palsy. Coordination was reduced bilaterally with finger-to-nose and fine finger movements. She had marked dysmetria with finger-to-nose and heel-to-shin movements. The initial MR imaging examination, performed 2 days after the onset of obtundation, demonstrated focal hyperintensities in the thalami, periaqueductal area, and caudates bilaterally on T2-weighted, FLAIR, and diffusion-weighted images (Fig 1A, -B). There was no obvious hyperintensity in the cerebral white matter, and no brain atrophy was found.
Axial FLAIR (A) and diffusion-weighted images (B) from the initial MR imaging examination both demonstrate hyperintensities in the thalami and caudates bilaterally. Axial FLAIR (C) and diffusion-weighted images (D) from the follow-up MR imaging examination 1 year and 10 months later show no abnormal signal intensities in the thalami and caudates bilaterally. Axial FLAIR image (E) shows that there is mild interval increased T2 hyperintense signal intensity in the frontal and parietal white matter.
The patient was given thiamine because of the suspected Wernicke encephalopathy. Her level of consciousness improved, and she became more alert and responsive. However, recent memory was severely reduced and remote memory was moderately reduced. She walked with assistance. She was discharged to a long-term care facility.
Follow-up MR imaging performed 22 months later demonstrated that the previous hyperintensities seen on T2-weighted, FLAIR, and diffusion-weighted images in the thalami, caudates, and periaqueductal region had disappeared (Fig 1C, -D). However, extensive and confluent minimally increased signal intensity throughout the cerebral white matter appeared on T2-weighted and FLAIR images. In addition, the follow-up imaging showed interval mild diffuse volume loss with an enlarged third ventricle and slightly prominent vermian atrophy (Fig 1E). At this point, the patient obtained alimentation using a gastrostomy tube. She continues to have poor mental status and lives in a long-term care facility. The neurologic examination revealed difficulty on tandem walking.
Case 2
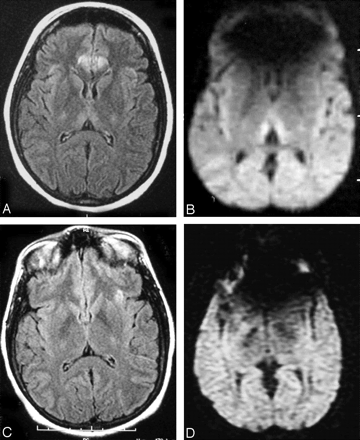
A 49-year-old man was brought to the emergency department in a stuporous state. He had started to have severe nausea and vomiting approximately 8 days before admission to the hospital. He had been an alcoholic for years and was malnourished. A neurologic examination disclosed nystagmus and ataxia of gait. On the initial MR examination, T2-weighted and FLAIR images showed minimal hyperintensities bilaterally in the thalami and mild hyperintensity in the periaqueductal area. Diffusion-weighted images revealed moderate hyperintensities in the thalami bilaterally, which were more obvious than those on T2-weighted and FLAIR images. However, no hyperintensity was found in the periaqueductal area on diffusion-weighted images. There was a minimal overall volume loss, with slightly enlarged sulci and ventricles (Fig 2A, -B). In addition, T2-weighted and FLAIR images showed minimal diffuse cerebral white matter hyperintensities. After the start of a thiamine infusion, his consciousness began to improve gradually. However, he continued to display persistent antegrade amnesia when discharged to a long-term care facility.
Axial FLAIR (A) and diffusion-weighted images (B) from the initial MR imaging examination both demonstrate minimal hyperintensities in the thalami bilaterally (arrows). The hyperintensities are more obvious on the diffusion-weighted image. T2 hyperintensity in the right frontal lobe represents encephalomalacia from a previous injury. Axial FLAIR (C) and diffusion-weighted images (D) from the follow-up MR imaging examination 9 months later show no abnormal signal intensities in the thalami bilaterally. Note the overall volume loss with enlarged sulci and ventricles.
The follow-up MR imaging examination, performed 9 months after the initial onset of neurologic symptoms, showed that the previous thalamic and periaqueductal hyperintensities seen on T2-weighted, FLAIR, or diffusion-weighted imaging had disappeared. Compared with the initial MR imaging examination, the ventricles and sulci increased in size, suggesting the development of brain atrophy (Fig 2C, -D). Diffuse cerebral white matter hyperintensities persisted on T2-weighted and FLAIR images. The patient still had problems with cognition and had severe tremors, dysmetria, and titubation. He continued to live in a long-term care facility.
Case 3
A 24-year-old woman was admitted because of bilateral progressive vision loss in a 10-day period. The patient had a medical history significant for obesity, for which she had undergone gastric bypass 2 months previously. Since that time, she had persistent nausea and vomiting and was unable to keep down solid foods, with a weight loss of 22.5 kg. A neurologic examination revealed unsteadiness of stance and gait with a positive Romberg sign, bilateral horizontal nystagmus, and absent tendon reflexes. The eye examination showed 20/400 and 20/200 vision. She also had bilateral optic disk edema and some retinal hemorrhages in the peripapillary region. The patient had previous normal findings on her eye examination, when her vision reportedly was 20/20 bilaterally. MR imaging showed the focal lesions in the thalami bilaterally, which were mildly hyperintense on T2-weighted and FLAIR images and markedly hyperintense on diffusion-weighted images. No abnormal signal intensity was found in the periaqueductal region. There was no brain atrophy or signal intensity change in the cerebral white matter (Fig 3A, -B).
Axial FLAIR (A) and diffusion-weighted images (B) from the initial MR imaging examination both demonstrate hyperintensities in the thalami bilaterally. The hyperintensities are more obvious on the diffusion-weighted image. Axial FLAIR (C) and diffusion-weighted images (D) from the follow-up MR imaging examination 9 months later show no abnormal signal intensities in the thalami bilaterally. No brain atrophy is found. The abnormal signal intensity in the bilateral front lobes on both the FLAIR and diffusion-weighted images results from the artifact. The follow-up diffusion-weighted image (D) was obtained only using a superoinferior gradient.
After thiamine treatment, her vision improved remarkably to the 20/25 and 20/20 level. Her gait improved significantly with mild recovery of deep tendon reflex. However, she continued to have horizontal nystagmus at the time of discharge.
The follow-up MR imaging performed 9 months later showed that the previous thalamic hyperintensities seen on T2-weighted, FLAIR, and diffusion-weighted images had disappeared. There was no other abnormal signal intensity. No brain atrophy was found (Fig 3C, -D). The neurologic examination showed that the patient still had some residual balance difficulty, especially when she was trying to multitask while walking. The eye examination showed only minimal residual optic atrophy bilaterally.
Discussion
MR imaging characteristics are unique and are often indispensable in making the diagnosis of Wernicke encephalopathy. The typical imaging findings in acute Wernicke encephalopathy include T2-weighted and FLAIR hyperintense areas that surround the third ventricle and aqueduct. In this study, imaging in all 3 patients in the acute stage showed thalamic hyperintensities on T2-weighted, FLAIR, and diffusion-weighted images. The thalamic hyperintensities on diffusion-weighted images in cases 2 and 3 were more marked than those on T2-weighted and FLAIR images. The clear demonstration of thalamic lesions is important for the diagnosis and follow-up because of the frequent involvement of the thalamus in Wernicke encephalopathy. In comparison to conventional imaging, therefore, diffusion-weighted imaging could offer a potential diagnostic advantage in some patients with Wernicke encephalopathy.
The pathologic changes that underlie acute Wernicke encephalopathy lesions are complex and consist of mechanisms that could lead to ischemic-like changes in the thalami (ie, cytotoxic edema or vasogenic edema) (4–9). The thalamic hyperintensities on diffusion-weighted images could be due to restricted water diffusion in the lesions.
In cases 2 and 3, diffusion-weighted images showed normal signal intensity in the periaqueductal area when moderate-to-marked hyperintensities were found in thalami. It might be just a reflection of the difference in the degree of edema. However, in case 2 of this study, FLAIR and T2-weighted images depicted a periaqueductal lesion. Moreover, we also previously experienced several cases in which the diffusion-weighted images poorly demonstrated lesions in the periaqueductal areas when lesions were well seen on FLAIR and T2-weighted images. Therefore, a possible difference in the pathologic mechanism between the thalamic and periaqueductal regions cannot be excluded (4, 7).
The follow-up imaging studies in the chronic stage showed no abnormal thalamic and periaqueductal intensities on the FLAIR, T2-weighted, and diffusion-weighted images. This finding suggests the disappearance of vasogenic and/or cytotoxic edema with time. However, the normalized thalamic and periaqueductal intensities were not well correlated with improvements of the clinical symptoms in these patients. Patient 2 still had ataxia, severe tremors, and dysmetria, and patients 1 and 3 still had problems with balance, though no thalamic and periaqueductal lesions were found in these 3 patients.
The brain volume loss in patients with Wernicke encephalopathy has been described as both a diffuse process and as more focal abnormalities involving the third ventricle, cerebral aqueduct, mammillary bodies, and the cerebellum vermis (3, 10–12). Cases 1 and 2 demonstrated volume loss. However, the volume loss was predominantly diffuse and not focal. The volume loss seen could be due to cachexia-induced brain shrinkage in these patients.
In case 1, increased T2 signal intensity appeared diffusely in the white matter on the follow-up T2-weighted and FLAIR images. The reason is unknown. Considering that neurons in Wernicke encephalopathy have pathologic findings that resemble anoxic damage (4, 7), we believe that the white matter could have diffusely suffered a similar insult with resulting changes in signal intensity. In case 2, diffuse white matter hyperintensity on T2-weighted images was found at both the acute and chronic stage. However, damage resulting from alcoholism might have occurred previously. Thus the hyperintensity at the acute stage could be a reflection of previous injury. Ethanol can adversely affect vascular, glial, and neural tissues and can also cause myelin degeneration (10). The diffuse white matter signal intensity change seen in the patient with chronic alcoholism could be the reflection of these pathologic changes.
Like patients 1 and 2, patient 3 continued to experience sequelae of the Wernicke encephalopathy. Unlike the other 2 patients, however, patient 3 had no diffuse signal intensity change on the follow-up MR examination. Moreover, volume loss was not found in case 3. This is possibly because the insult that caused the Wernicke encephalopathy, nutritional deficiency, in case 3 occurred for a shorter duration and was less severe than that in the other 2 patients.
In summary, we present the case reports of 3 patients with Wernicke encephalopathy in which conventional MR imaging and diffusion-weighted imaging were performed at the acute and chronic stages. The resolved signal intensity abnormalities of the focal Wernicke encephalopathy lesions with time did not directly relate to the clinical symptoms on follow-up examinations. Brain atrophy and diffuse signal-intensity changes in cerebral white matter can be imaging features in the chronic stage of Wernicke encephalopathy. Their presence or absence could be related to the length of clinical history and other comorbidities. In addition, diffusion-weighted imaging provided an advantage in depicting thalamic lesions and could be helpful in diagnosing Wernicke encephalopathy.
References
- Received November 28, 2003.
- Accepted after revision January 10, 2005.
- Copyright © American Society of Neuroradiology