Abstract
BACKGROUND AND PURPOSE: Despite advances in mechanical thrombolysis for acute stroke, recanalization rates remain approximately 50%–60%. Technologic improvements allowed safe intracranial delivery of stents. To study the feasibility of stent-assisted recanalization for acute stroke, we deployed self-expanding or balloon-mounted stents in 2- to 3.5-mm canine vessels acutely occluded with clot emboli.
METHODS: Six mongrel dogs were placed under general anesthesia. A guiding catheter was placed in the distal vertebral artery or an external carotid artery branch. A 7 × 3 mm (length × diameter) soft or hard clot was injected into the catheter and allowed to embolize distally; 20 vessels were successfully occluded. After systemic heparin anticoagulation, recanalization with a self-expanding stent was attempted in 11 vessels (5 occluded with soft clot; 6, with hard clot). Balloon-mounted stents were placed in an attempt to revascularize 9 vessels (4 occluded with soft clot; 5, with hard clot). Vessel recanalization was assessed as the primary end point. Side-branch occlusion and stent-induced vasospasm were also assessed.
RESULTS: Thrombolysis in Myocardial Infarction/Thrombolysis in Cerebral Infarction flow for 11 vessels treated with self-expanding stents versus 9 treated with balloon-mounted stents was as follows: grade 3, 91% of vessels versus 78% of vessels; grade 2, 0% versus 11%; grade 1, 9% versus 0%; grade 0, 0% versus 11%. Lower rates of spasm and side-branch occlusion were noticed with self-expanding stents. Grade 2/3 flow was achieved in 18/20 vessels (90%).
CONCLUSIONS: Excellent recanalization was demonstrated with both stents. Recanalization in self-expanding stents was achieved without pre- or post-balloon dilation. Stents may prove to be a useful adjunct for intra-arterial acute stroke treatment.
More than 700,000 strokes occur annually in the United States, resulting in a cost of more than $50 billion in health care expenditures and lost productivity.1 Until recently, the only approved treatment for acute stroke was intravenous tissue plasminogen activator (tPA) delivered within the first 3 hours of stroke onset.2 Unfortunately, most individuals experiencing a stroke do not present for medical attention within the first 3 hours of symptom onset. Additionally, patients with National Institutes of Health Stroke Scale scores greater than 10 have decreased odds of recovery, compared with those having less severe strokes.3 Furthermore, current data have shown tPA to be toxic to neurons and endothelial cells.4 Intra-arterial delivery of thrombolytic agents into occluded middle cerebral arteries within 6 hours of symptom onset in the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial resulted in recanalization rates of 66%.5 Despite overall improvement in clinical outcome, an 11% rate of early symptomatic intracranial hemorrhage was experienced by the treatment group.
Use of the Merci device (Concentric Medical, Mountain View, Calif), a mechanical embolectomy device recently approved by the Food and Drug Administration to remove blood clots from neurovascular vessels for acute stroke therapy, resulted in recanalization in 69 of 151 (46%) patients on an intention-to-treat basis and in 68 of 141 (48%) patients in whom the device was actually deployed.6 These statistics included 17 patients in whom the artery was opened with the device and tPA was infused into distal vascular branches not accessible with the device. The addition of thrombolytics increased recanalization results with the Merci device from 33% to 51% but was associated with increased intracranial hemorrhage rates.7
Despite the addition of intra-arterial thrombolytics in conjunction with mechanical thrombolysis with devices such as the Merci clot retriever, recanalization rates remain unacceptably low. Considerably higher recanalization rates (89%–93%) have been achieved in peripheral and coronary vessels in which stent implantation or stent-assisted angioplasty has been used.8 Although coronary and peripheral vessel occlusions typically result from underlying high-grade stenotic lesions, whereas intracranial vessel occlusions are primarily embolic in origin, stent-assisted revascularization of acute intracranial vessel occlusions has previously been described.9–11 In the setting of acute occlusive stroke, we surmise that the stent morselizes and displaces clot outside the parent vessel lumen, thus restoring flow. The stent struts (for balloon-mounted stents) act as a buttress, preventing clot from herniating back into the vessel lumen after displacement by the stent. To determine the feasibility of stent-placement technique for revascularization, we used an in vivo model to assess recanalization rates after stent placement of vessels acutely occluded with thrombotic emboli.
Materials and Methods
A prospective study was designed to examine stent placement to achieve recanalization of acute embolic occlusion in the canine cerebral vasculature. This study was approved by the Animal Care and Use Committee at the University at Buffalo and was conducted in accordance with guidelines established by the Animal Welfare Act.12
Six mongrel dogs (each weighing at least 20 kg) were used in this study. Canines were chosen for their anatomic characteristics with respect to vessel size and vessel response to stent placement (degree of vasospasm similar to that in human vessels). After a general anesthesia agent had been delivered endotracheally with intubation, femoral artery access was obtained, and a 6F guiding catheter was placed in the target vessel, either the distal vertebral artery or a branch of the external carotid artery, by using a 0.035-inch hydrophilic wire. A clot of soft (9 vessels) or hard (11 vessels) consistency, composed of citrated porcine blood (proprietary clot-making process; Boston Scientific), was injected into the catheter and allowed to embolize distally. Soft- and hard-consistency clots were achieved by changing in vitro flow parameters during clot preparation. The in vitro flow parameters are changed by increasing the shear rate on the blood (ie, higher shear rate yields harder clot and lower shear rate yields a softer clot).
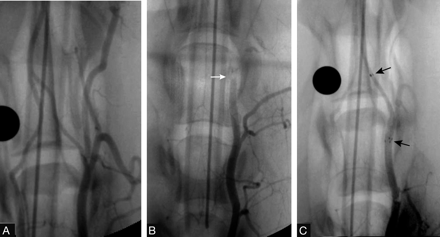
On the basis of the Boston Scientific experience in making and determining clot consistency, soft- and hard-consistency grading was subjectively assigned. (Boston Scientific has used this process of clot making for more than 3 years to evaluate acute ischemic stroke devices.) The soft- and hard-clot consistency was also verified by the researchers at University at Buffalo Neurosurgery Department.13 The 20 target vessels measured 2–3.5 mm in diameter, similar to the size of human middle cerebral, basilar, and distal intracranial internal carotid arteries. Each clot was approximately 7 mm in length and 3 mm in diameter. Vessel occlusion was documented by digital subtraction angiography (Fig 1 A, -B) and assessment of a Thrombolysis in Myocardial Infarction (TIMI)14 or a Thrombolysis in Cerebral Infarction (TICI) flow grade of 0 or 115,16 (persistent occlusion or trickle flow).
A, Baseline angiographic view of the canine distal vertebral arteries at the level of the foramen magnum, proximal to the vertebrobasilar junction. B, Angiographic view documenting complete vessel occlusion (arrow) achieved by injecting thrombus through the guiding catheter. C, Angiographic view confirming restoration of flow due to stent-assisted recanalization following deployment of the Wingspan. Arrows denote proximal and distal ends of the stent.
Therapeutic systemic anticoagulation was achieved by administering sufficient heparin (2500–3000 U) to produce an activated coagulation time of greater than 250 seconds. A 0.014-inch microwire was then advanced through a microcatheter past the occluded segment. A preselected balloon-mounted (or balloon-expandable) stent (Liberté, Boston Scientific, Natick, Mass) or a self-expanding stent (Wingspan, Boston Scientific/Target, Natick, Mass) was advanced over the wire to the occluded segment of the vessel and deployed. The Liberté is a new-generation balloon-expandable stent. The Wingspan is a self-expanding stent that was chosen because it exerts a greater outward force than a Neuroform intracranial stent (Boston Scientific).17 For these experiments, we wanted to assess the capabilities of these stents to recanalize embolic occlusion because they are flexible highly navigable devices capable of accessing the human intracranial vasculature. The deployment of either stent was accomplished within 20 minutes of vessel occlusion. No thrombolytics were administered. No mechanical manipulations were attempted with the microwire, snare, or retriever device. If a balloon-mounted stent was used, slow-inflation techniques were practiced.18 The balloon-mounted stent was deployed by using approximately 6 atm of pressure. No pre- or post-balloon dilation was used with the self-expanding or balloon-expanding stent.
Following stent deployment, angiography was performed to assess the degree of recanalization (defined according to TIMI/TICI flow grades of TIMI 2/TICI 2a or b [partial perfusion] or TIMI/TICI 3 [complete perfusion]) and the presence or absence of side-branch occlusion (Fig 1C). Vasospasm of the parent vessel was also assessed by angiography. Each angiographic image was evaluated independently by 2 investigators (E.I.L., R.A.H.). The animals were euthanized with pentobarbital (100 mg/kg).
Results
Recanalization with self-expanding stents was attempted in 5 vessels occluded with soft clot and 6 vessels occluded with hard clot. Balloon-mounted stents were used to revascularize 4 vessels occluded with soft clot and 5 vessels occluded with hard clot. Subjectively, the self-expanding stent delivery system was easier to navigate than the balloon-mounted system. Pre- and post-balloon dilations were not necessary with either stent system for stent placement of either soft- or hard-consistency clot. The balloon-mounted stent was deployed by using approximately 6 atm of pressure. Irrespective of type of clot or stent delivery system, a 100% success rate was achieved in crossing the occlusion site.
Postprocedure TIMI/TICI flow grades for the 20 vessels treated with stents are provided in Table 1. Recanalization (TIMI/TICI 2 or 3) was achieved in 8 of 9 vessels treated with balloon-mounted stents and in 10 of 11 vessels treated with self-expanding stents, for an overall recanalization rate of 90%. Self-expanding stents incurred lower rates of vasospasm and side-branch occlusion than balloon-mounted stents. Findings of vasospasm and side-branch occlusion did not affect recanalization grade. Neither stent had an influence on TIMI/TICI recanalization grade. The results are summarized in Table 2.
TIMI/TICI flow grades before and after stent placement
Stent used, clot consistency, and initial TIMI/TICI flow related to post-stent recanalization rate and findings
Discussion
Nearly 8 years after the completion of PROACT II, recanalization rates for occluded intracranial vessels have not improved. During this same period, tremendous advances in stent technology have taken place, including the development of a self-expanding stent intended for the treatment of aneurysms in the intracranial circulation (ie, the Neuroform stent). Self-expanding stent technology was born out of the necessity to deliver stents through the tortuous anatomy often encountered intracranially. Balloon inflation is not required for self-expanding stent deployment, thus the risk of aneurysm or parent vessel rupture is minimized. Additionally, vessel barotrauma is minimized with self-expanding stents versus balloon-mounted stents, which require 6–8 atm of pressure for deployment. Stent technology and balloon technology evolved at a ferocious pace as these devices became more deliverable with lower profiles, lower metal-to-artery ratios, and lower pressures required for deployment.
With industry focused on the advancement of stent technology for the treatment of acute coronary occlusion, perhaps this technology could be applied intracranially for acute intracranial vascular occlusions. If stents could be delivered safely into intracranial vessels, could they be used to morselize and buttress clot outside the parent vessel lumen, thus restoring flow? Although experience with 2 cases10,11 provided anecdotal evidence in support of this premise, we conducted an in vivo feasibility study to answer this question. As demonstrated from the results presented here, excellent rates of revascularization were achieved with self-expanding and balloon-mounted stents. These rates were higher than those achieved with the prevailing standard of mechanical thrombolysis, the Merci retriever. Self-expanding stents tended to induce less vasospasm, compared with balloon-mounted stents.
Although vessel patency at the occlusion site is a primary consideration in the context of acute stroke therapy, it is also important to determine whether distal embolization or side-branch occlusion, with or without infarction, has occurred. A significant advantage of recanalization accomplished with stent assistance over that with clot retrieval devices, such as the Merci retriever, is that access to distal vessels is not compromised. When one uses clot-retrieval devices, access to the target lesion is surrendered when the retriever is removed. Should the clot persist, steps must be repeated to regain access to the target lesion, which is a process that is both time-consuming and associated with risk in the setting of an acute stroke. Using a stent-assisted recanalization technique, one maintains wire access at all times, minimizing both time and risk associated with treatment of the occlusion. In addition, lytic agents could be infused into occluded side branches. In the canine data presented here, side-branch occlusion resulting from “snowplowing” of clot into side branch orifices did not affect recanalization rates. We do not know the likelihood of or significance of jailing of small branches and/or in-stent stenosis associated with the use of stents for acute stroke revascularization in the clinical setting. With the Wingspan, preliminary clinical data show in-stent stenosis of 10% for intracranial atherosclerosis.19 In acute stroke, however, the main objective is immediate restoration of blood flow.
An issue pertinent to the use of stents as an adjunct for intra-arterial stroke treatment is the need for antiplatelet therapy. In the preliminary clinical experience,10,11 a bolus dose (180 mcg/kg) of eptifibatide was administered intravenously at the time of treatment, and then clopidogrel was prescribed for a minimum of 1 month. This protocol likely increases the risk of hemorrhagic complications associated with revascularization, but not more so than tPA thrombolysis, because thrombolytic agents may be more likely to cause hemorrhage than antiplatelet agents. As shown by previous trials, mechanical thrombolysis may reduce the need for incremental doses of thrombolytics, thus minimizing the hemorrhage risk.6,7
Conclusions
In this canine in vivo model of acute embolic occlusion, the feasibility of stent deployment to achieve vessel patency was examined. TIMI/TICI grade 2 or 3 revascularization was achieved in 90% of vessels treated with self-expanding and balloon-expandable stents. Revascularization with self-expanding stents was achieved without pre- or post-balloon dilation. Stent-induced vasospasm was appreciated more commonly after the deployment of balloon-mounted stents as was side-branch occlusion. Further investigation in clinical studies is warranted to determine the effectiveness of stent technology for acute embolic stroke.
Acknowledgments
We thank Michael F. Rejewski, BA, and Ann Marie Paciorek, BS, for care and preparation of the animals and Paul H. Dressel for preparation of the illustrations.
Footnotes
Author contributions: Levy—study design and writing; Sauvageau—study experiments and conceptual design; Hanel—experiments and conceptual design; Parikh—experiments and conceptual design; Hopkins—conceptual design and review.
This study was supported by Boston Scientific. Dr. Hanel received research grant support from Boston Scientific Corporation. Dr. Hopkins has a financial interest in, serves as a consultant for, and has received research grant support from Boston Scientific Corporation. Dr. Levy has received research grant support and honoraria for symposia from Boston Scientific Corporation. Mr. Parikh was an employee of Boston Scientific Neurovascular at the time this work was conducted.
References
- Received November 7, 2005.
- Accepted after revision February 3, 2006.
- Copyright © American Society of Neuroradiology