Abstract
BACKGROUND AND PURPOSE: The cause of “posterior reversible encephalopathy syndrome” (PRES) is not established. We recently encountered several patients who developed PRES in the setting of severe infection. In this study, we comprehensively reviewed the clinical and imaging features in a large cohort of patients who developed PRES, with particular attention to those with isolated infection, sepsis, or shock (I/S/S).
METHODS: The clinical/imaging features of 106 patients who developed PRES were comprehensively evaluated. In 25 of these patients, PRES occurred in association with severe I/S/S separate from transplantation. The clinical/imaging features (computer tomography, MR imaging, and MR angiography [MRA]) of the patients with I/S/S were further evaluated, including organ/tissue/blood culture results, mean arterial blood pressure (MAP) at toxicity, extent of cerebral edema, and presence of vasospasm.
RESULTS: PRES occurred in association with I/S/S in 25 of 106 patients (23.6%), in addition to 4 other major clinical settings, including cyclosporine/FK-506 (post-transplant) neurotoxicity (46.2%), autoimmune disease (10.4%), postchemotherapy (3.7%), and eclampsia (10.4%). In the 25 patients with I/S/S, available cultures demonstrated a predominance of gram-positive organisms (84%). Blood pressure was “normal” at toxicity in 10 patients (MAP, 95 mm Hg); “severe” hypertension was present in 15 patients (MAP, 137 mm Hg). Extent of brain edema graded on imaging studies was greater in the normal MAP group compared with the severe hypertension group (P < .05). MRA demonstrated vasospasm in patients with severe hypertension and vessel “pruning” in the normal MAP group.
CONCLUSION: Infection/sepsis/shock may be an important cause of PRES, particularly in relation to infection with gram-positive organisms.
The imaging features of eclampsia and cyclosporine/FK-506 neurotoxicity seen after allogeneic bone marrow transplantation (allo-BMT) are similar. CT and MR imaging demonstrate cortex/white matter vasogenic edema in the parietal/occipital regions with less frequent frontal, temporal-occipital, and cerebellar involvement.1–4 Isolated reports have demonstrated vasospasm at MR angiography (MRA) in both of these conditions.2,5–7 This imaging pattern has been identified in patients with systemic conditions, such as nonspecific renal inflammatory disease (glomerulonephritis, hepatorenal syndrome), systemic lupus erythematosus (SLE), Wegener granulomatosis, or postchemotherapy.8–15 The term “posterior reversible encephalopathy syndrome” (PRES) is commonly used in these patients focusing on the similarity in imaging appearance, in particular the common parietal/occipital distribution of the abnormality.12–15
We recently identified several patients who developed PRES in conjunction with severe infection or sepsis. The purpose of this study was to retrospectively evaluate the clinical conditions surrounding the development of PRES with particular attention to the frequency of association with infection, sepsis, or shock (I/S/S).
Materials and Methods
The radiology report data base at our institution was searched (Jan 1998–Aug 2005) for any patients where PRES or posterior reversible encephalopathy was cited in brain MR imaging reports. Further similar data base searches were performed for reference to cyclosporine neurotoxicity, tacrolimus/FK-506 neurotoxicity, SLE, Wegener granulomatosis, scleroderma (systemic sclerosis), hypertensive encephelopathy, preeclampsia, and eclampsia. Brain MR imaging studies along with CT imaging studies were reviewed in the identified patients for features consistent with the characteristics of cyclosporine/FK-506 neurotoxicity, eclampsia, or PRES.
Patients with imaging features consistent with PRES were tabulated combined with the PRES neurotoxicity data base belonging to one of the authors (Jan 1991–June 2002), and the resulting data were pooled. The combined data represent the spectrum of cases with PRES neurotoxicity for this report. Institutional Review Board approval was obtained for this retrospective study.
One hundred and six patients were identified who developed neurotoxicity and brain imaging consistent with the previous literature description of CsA/FK-506 neurotoxicity, eclampsia, or PRES. Criteria included complete or partial expression of the typical PRES pattern, complete or partial reversibility on follow-up imaging, or vasogenic edema as demonstrated by MR diffusion imaging. In 25 of these 106 patients, neurotoxicity and PRES developed in the setting of severe infection, sepsis, or shock. This report briefly reviews the clinical background of the 106 patients in the overall population and specifically focuses on clinical and imaging features encountered in the 25 patients with infection, sepsis, and shock.
Clinical Evaluation
The clinical inpatient and outpatient records of these patients were reviewed. Specific attention was paid to identify clinical features leading up to and surrounding the development of PRES along with known associations including CsA/FK-506 neurotoxicity and eclampsia. The presence or absence of hypertension, CsA/FK-506 levels, evidence of endothelial injury and hemolysis (lactate dehydrogenase [LDH], platelet levels, red cell fragmentation [similar to BMT thrombotic microangiopathy]), evidence of immune system dysfunction (autoantibody formation, graft-versus-host effects, and organ rejection), liver function, renal function, pulmonary function, and presence of infection or sepsis were sought and tabulated. Where more than one clinical association was present, the dominant clinical association was used for tabulation.
In patients with infection, sepsis, and shock, evidence of coexistent “multiple organ dysfunction syndrome” (MODS) was sought and tabulated with guidance from the “sepsis-related organ failure assessment” (SOFA) score as developed by the European Society of Intensive Care Medicine 1994 consensus meeting.16,17 Parameters used by this score are designed to identify in a simple and concise fashion evidence of developing organ dysfunction/failure in the presence of sepsis including: coagulation, pulmonary, hepatic, renal, cardiovascular, and neurologic systems. Evidence of organ failure was sought in 4 of these systems (coagulation: drop in platelet count [<150 × 103/mm3]; pulmonary: respiratory failure or hypoxemia not related to pneumonia; liver: bilirubin elevation [>1.2 mg/dL]; renal: creatinine elevation [>1.2 mg/dL]) and tabulated. Neurologic dysfunction is intrinsically reflected in PRES neurotoxicity and cardiovascular dysfunction reflected in associated hypertension. Mean arterial pressure (MAP) at toxicity was calculated in a standard fashion (MAP = 2/3 diastolic + 1/3 systolic pressure).
Imaging Evaluation
CT was the sole imaging study in 18 patients with MR imaging available at toxicity in 88 patients (including comparison CT and follow-up MR imaging studies). CT studies were obtained with 5-mm section thickness through the posterior fossa along with 5–10-mm section thickness through the supratentorial hemispheres. Contrast material when used consisted of intravenous 150 mL iothalamate meglumine (Conray 60; Mallinckrodt, St. Louis, Mo) or iohexol 300 (GE Medical Products, Milwaukee, Wis).
MR imaging, where obtained, was performed at 1.5T including sagittal and axial T1-weighted images (TR/TE/section width/NEX, 600 ms/minimum/5 mm/1) with 5-mm section thickness and spin-echo or fast spin-echo axial proton attenuation (TR/TE/section width/NEX, 2000–2500/minimum/5 mm/1) and T2-weighted images (TR/TE/section width/NEX, 2500–3000/84–102effective/5/1). Contrast-enhanced T1-weighted images were obtained with 0.1 mmol/kg gadolinium dimeglumine (Magnavist; Berlex Laboratories, Wayne, NJ) or gadoteridol (Prohance; Bracco Diagnostics, Princeton, NJ) using typical T1-weighted parameters as described above. Fluid-attenuated inversion recovery (FLAIR) images (TR/TE/TI, 9000–10,000 ms/149 ms/2200 ms) and diffusion-weighted imaging (DWI; single-shot echo-planar; TR/TE/section width/matrix, 10,000 ms/minimum/5 mm/128) sequences were also available in most patients.
Imaging Features of CsA/FK-506 Neurotoxicity, Eclampsia, and PRES
The scope of features seen in CsA/FK-506 neurotoxicity, eclampsia, and the PRES imaging appearance has been described previously.1–4,9–15 The locations of the regions of imaging abnormality were itemized and tabulated. Specific regions were tabulated separately, including frontal lobe, parietal region, occipital lobe, temporal lobe, cerebellum, brain stem, basal ganglia, and deep white matter. Features of involvement were further characterized as patchy, confluent, or linear in appearance. The presence or absence of lesion enhancement was noted and tabulated. DWI features (normal/restricted) were identified. The presence of focal areas of brain infarction with restricted diffusion or regions of brain hemorrhage were itemized and tabulated.
Grading of Vasogenic Edema in PRES
In the patients with I/S/S, CT or MR imaging studies were graded for extent and severity of cortex and white matter vasogenic edema. Extent and severity of edema in the involved regions were independently assessed by 2 observers blinded to patients’ blood pressure, studies were graded on a 5-point scale summarized in Table 1, and results were tabulated for each patient. Difference in patient grade was agreed upon by consensus. Edema grade results for patients with severe hypertension and without hypertension were separately averaged and compared.
Vasogenic edema scale
Vascular Assessment
In 11 patients with I/S/S, MRA was available along with MR imaging in the time frame of neurotoxicity and PRES. MRA was obtained using 3D time-of-flight (TOF) technique (TR/TE/Flip angle/FOV/matrix/acquisitions, default/min/45°/18–22 cm/226 × 224/1) with multiple overlapping slab reconstruction. In 10 of 11 patients, MRA was obtained during initial MR assessment of neurotoxicity, and in 1 patient, MRA was obtained on a follow-up study 1 week after toxicity and after the patient had clinically stabilized.
MRA studies were evaluated for the presence or absence of vascular abnormality or vasospasm. Studies were blindly and independently graded by 2 neuroradiologists, and any differences were resolved by consensus. Traditional features of vasospasm or vasculitis were identified including: significant diffuse constriction of first-, second-, and third-order branch vessels, areas of focal vessel narrowing and constriction, and string-of-bead appearance.
Statistical Assessment
Statistical significance was evaluated using the SAS software package, PROCAPABILITY statistical analysis software function (SAS release 8.2; SAS Institute, Cary NC). Comparison between hypertensive and nonhypertensive subsets was performed with Student t test and Wilcoxon signed rank test. Statistical significance was considered to exist for P < .05.
Results
The clinical background of the 106 patients is summarized in Table 2. Seventy-two patients (67.9%) were female and 34 (32.1%) were male; their average age was 42.4 years (range, 17–79 years). Headache, vision change, altered mental status, nausea, or aphasia (alone or in combination) was the presenting symptom in 35 (33%) of patients and seizure (frequently accompanied by or preceded by headache or vision change) in 71 (67%). Blood pressure was normal (patient baseline) at presentation in 32 patients, slightly elevated in 11 patients, and severe in 63 patients.
Categories and timing of PRES neurotoxicity
Overall Patient Clinical Profile
In 49 (46.2%) patients, neurotoxicity developed in association with cyclosporine/FK-506 immune suppression. Transplantation was present in 46 of 49 patients (solid organ, 20; allo-BMT, 26), and 3 patients received cyclosporine for treatment of marrow disease (pure red cell aplasia, aplastic anemia). Four (3.8%) patients developed PRES in association with cancer chemotherapy and in 11 (10.4%) patients, PRES occurred in association with autoimmune disease (SLE, 5 patients and 1 patient each with Wegener granulomatosis, scleroderma, polyarteritis nodosa, psoriasis, Graves disease, and rheumatoid factor positive arthropathy). PRES was associated with eclampsia or delayed eclampsia in 11 (10.4%) patients.
In 4 patients, PRES developed in association with increasing or acute hypertension with either chronic renal disease (nephrosclerosis; chronic renal failure and dialysis) or no obvious cause of acute hypertension (chronic drug use; prior renal cell carcinoma). In 2 patients, no specific cause was identified with known but unchanged chronic hypertension. In 25 (23.6%) of 106 patients, neurotoxicity and PRES were noted to occur in association with I/S/S.
PRES in Infection, Sepsis, and Shock
In 23 patients, significant infection and/or bacteremia occurred in close association with the development of PRES. In 2 additional patients, PRES developed after an episode of severe hemorrhagic shock. In 11 of the 23 patients with infection, clinical sepsis (sepsis, severe sepsis, or septic shock) was noted or suspected during their illness before development of PRES. The clinical profile and characteristics of the patients with infection, sepsis, and shock are summarized in Table 3.
Clinical features of patients with infection, sepsis, and shock
In 21 of these 23 patients with infection, PRES developed immediately after or coincident with the severe infection or bacteremia. In 18 of these 21 patients, PRES occurred within 2 weeks of the infection and in 3 patients (1 each: abscess, wound infection, blast crisis with sepsis), neurotoxicity developed between 20 and 30 days of infection identification (overall average, 6.7 days; range, 0–30 days). In 19 of the 21 patients, organ or tissue infection was present, and in 2 patients, isolated bacteremia and sepsis occurred. Of the 19 patients with organ/tissue infection, 12 patients also demonstrated bacteremia coincident with the PRES event. In 4 of the 19 patients with organ/tissue infection, documented infection was present but primary site cultures were not available due to ongoing antibiotic treatment. In 2 of the 23 patients with infection, timing was difficult to assess because of disease chronicity (1 patient with diabetes, chronic septic arthritis [Streptococcus aureus] requiring frequent debridement and multiple prior episodes of bacteremia developed neurotoxicity during renal dialysis; 1 patient with sickle cell disease where PRES occurred with high white count, repeat sickle cell crisis, and heavy rectal enterococcal colonization but 10 weeks after pneumonia with documented bacteremia). Four of 23 patients with infection/sepsis also received chemotherapy.
Organ or Tissue Infection
Organ/tissue culture results were available in 16 of 23 patients (15 of 21 patients coincident with PRES; 1 patient with chronic septic arthritis). These are reviewed in Table 3. In 10 infections, a single organism was isolated, and in 6 infections, mixed flora was present. In single-organism infections, gram-positive cocci were present in 8 of 10 and where mixed flora was cultured, at least one of the organisms was gram-positive. Gram-positive cocci were isolated, therefore, in 14 of 16 primary site infections.
Blood Cultures and Septicemia
Blood cultures were positive in 14 of the 21 patients, where infection was coincident with PRES (2 with primary bacteremia/sepsis, 12 with organ or tissue infection) as well as the 1 patient with sickle cell disease, sickle cell crisis, and pneumonia. The results are summarized in Table 3. In 10 patients, a single blood culture was positive coincident with PRES, and in 4 patients, multiple blood cultures were positive coincident with toxicity. Similar to the results of tissue and organ infection, most organisms identified (12 of 15) were gram-positive cocci.
Multiple Organ Dysfunction Accompanying I/S/S and PRES
In 18 of the 25 patients with I/S/S, evidence of evolving multiple organ dysfunction was identified coincident with the development of neurotoxicity/PRES. New or worsening organ dysfunction was documented in the systems typically involved in MODS including: coagulation dysfunction with platelet consumption (14 patients), pulmonary dysfunction separate from pneumonia (11 patients), liver dysfunction (9 patients), and renal dysfunction (12 patients). Two or more systems were abnormal and changing coincident with PRES in 15 patients (4-system dysfunction: 5 patients; 3-system dysfunction: 4 patients; 2-system dysfunction: 6 patients) with single-system dysfunction noted in 3 patients.
In 5 of 15 patients, manual peripheral smears were available at the time of toxicity and were abnormal demonstrating red cell fragmentation. In 8 of 9 patients, LDH levels obtained at toxicity were elevated and in 3 of these patients, peripheral smears were available and demonstrated red cell fragmentation consistent with hemolysis. In 7 of the 8 patients with elevated LDH, a significant decline in platelet count was also noted suggesting a consumptive coagulopathy and endothelial injury.
Blood Pressure
Average baseline blood pressures and blood pressure at the time of toxicity are listed in Table 3. Two separate groups of patients were identified. Group 1 (referred to as “normotensive”): In 10 patients (40%), blood pressure was either normal/patient’s baseline at toxicity (6 patients) or demonstrated only mild systolic pressure elevation relative to patient baseline at toxicity (4 patients). Group 2 (referred to as “severe hypertensive”): In 15 patients (60%), severe hypertension was present with significant elevation of systolic pressure (>200 mm Hg), diastolic pressure (>100 mm Hg), or both. MAP at toxicity in group 1 was 95 mm Hg (range, 78–106 mm Hg) and MAP at toxicity in group 2 was 137 mm Hg (range, 123–157 mm Hg).
Imaging Features
The imaging features of patients who developed neurotoxicity/PRES coincident with I/S/S are reviewed in Tables 4 and 5 and Figs 1–6. Patients demonstrated regions of vasogenic edema in typical locations reported for PRES, including cerebellum, temporal lobes, occipital region, parietal region, and frontal lobes. Unusual lesion locations were noted in 6 patients, including brain stem (medulla, pons, or midbrain), thalamus, and caudate nucleus. In 17 patients, DWI was available at 1 of the initial imaging evaluations. DWI was normal without restricted diffusion in 15 of 17 studies with 2 patients demonstrating areas of focal restricted diffusion along with focal areas of hemorrhage.
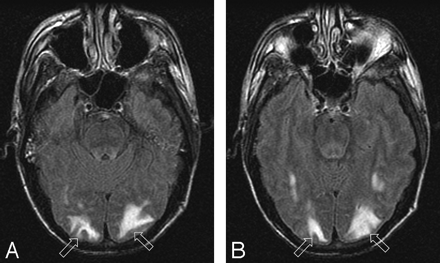
Patient 1 is a 39-year-old man with baseline blood pressure 122/61 mm Hg who had severe pneumonia with bronchial obstruction. Bronchial lavage grew Staphylococcus aureus and blood culture grew coagulase-negative staphylococci. Neurotoxicity developed 13 days after positive cultures with severe headache followed by a seizure with blood pressure at toxicity 118/70 mm Hg.
A–B, Brain MR imaging (FLAIR sequence) demonstrates moderate signal intensity abnormality from vasogenic edema in the occipital lobes bilaterally (open arrows) typical of the PRES pattern with full extension to the ventricular surface and moderate local cortical mass effect judged grade 3. Follow up imaging was not obtained, but the patient’s symptoms resolved completely.
Patient 7 is a 68-year-old woman with necrotic pancreatitis, a pancreatic abscess, and baseline blood pressure of 141/67 mm Hg. Abscess grew mixed flora with coagulase-negative staphylococci and Acinetobacter baumannii and blood culture was positive for coagulase-negative staphylococci. Altered mental status with PRES developed 7 days after positive cultures with blood pressure at toxicity of 168/68 mm Hg.
A-B, Brain MR imaging (FLAIR sequence) demonstrates vasogenic edema in the occipital (open arrows) and parietal region (curved arrows) bilaterally typical of PRES with extension into the deep white matter but no extension to the ventricle surface judged grade 2.
C-D, Brain MR imaging (FLAIR sequence) obtained 1 month after initial imaging and toxicity demonstrates near complete resolution of the edema in the occipital (open arrows) and left parietal region (curved arrow) with complete resolution in the right parietal area (arrow).
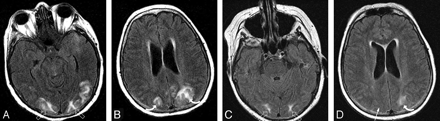
Patient 4 is a 56-year-old woman with a baseline blood pressure of 156/68 who developed a thigh abscess with culture growing mixed flora (Klebsiella pneumonial and enterococci). She developed MOD (coagulopathy, acute respiratory distress syndrome, acute renal failure, liver failure, and shock liver). On day 27 of intensive treatment of her infection and multiorgan failure, the patient developed altered mentation followed by a generalized seizure and blood pressure of 164/75 mm Hg.
A-D, Brain MR imaging (FLAIR sequence) obtained the 1-day after neurotoxicity demonstrates severe and extensive vasogenic edema primarily involving the subcortical white matter of the parietal (curved arrows), occipital (open arrows), and temporal lobe regions (arrowheads) bilaterally with ventricular distortion from edema judged grade 5.
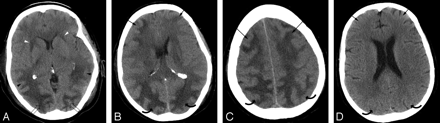
Patient 8 is a 55-year-old woman with multiple liver metastases from renal cell carcinoma who underwent liver wedge resection and intraoperative chemotherapy infusion. Baseline blood pressure was 115/70 mm Hg. She developed ARDS and Streptococcus pneumoniae sepsis 3 days after resection followed by pneumonia (S aureus) and line sepsis (coagulase-negative staphylococci). Patient developed altered mental status 3 days after pneumonia and bacteremia with blood pressure at toxicity of 150/80 mm Hg.
A-C, Brain CT images obtained at toxicity demonstrate vasogenic extensive edema in the occipital (open arrows), parietal (curved arrows), and frontal regions (arrows) bilaterally with focal edema also noted in the anterior limb internal capsule bilaterally (arrowheads) consistent with PRES with ventricular compression and deformity from the edema judged grade 5.
D, Follow-up brain CT imaging performed 1 month after initial imaging demonstrates complete reversal of the PRES pattern shown here in the parietal (curved arrows) and frontal (arrows) regions bilaterally.
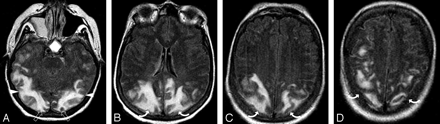
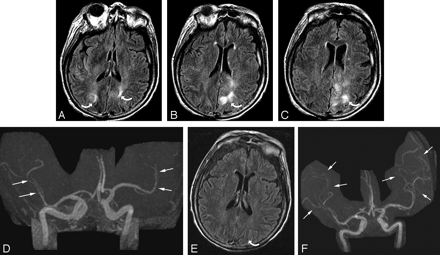
Patient 2 was a 73-year-old woman who had undergone gastric surgery. Her baseline blood pressure of 157/77 mm Hg. She developed aspiration and pneumonia. Bronchial washings and blood cultures grew Pseudomonas aeruginosa. Neurotoxicity developed 6 days after positive cultures with altered mental status and blood pressure 128/80 mm Hg.
A-C, Brain MR imaging (FLAIR sequence) obtained at the time of toxicity demonstrates an unusual pattern of vasogenic edema in the parietal region bilaterally much greater on the left (curved arrows) involving both cortex and some extension to the deep white matter judged grade 2.
D, MRA obtained at the same time as imaging demonstrates diminutive severely “pruned” intracranial vessels, in particular MCA branches (arrows).
E, Follow-up brain MR imaging (FLAIR sequence) obtained 1 month after initial imaging demonstrates complete resolution of the vasogenic edema bilaterally shown here only on the left (curved arrow). Incidental subdural hygromas are also present.
F, Follow-up MRA also obtained 1 month after initial imaging demonstrates marked improvement in vessel visualization with partial reversal of the severe “pruning” and spasm in the MCA branches (arrows) bilaterally. The patient’s mental status completely normalized.
Patient 19 was a 54-year-old woman who had undergone gastric bypass surgery. She developed a severe pneumonia 2 months after surgery that eventually required intubation along with antibiotic treatment. This occurred while she was being treated at an outside hospital. The patient developed vision changes, confusion, and hypertension (200/100 mm Hg) during treatment with initial CT imaging reported as negative, and she was transferred to our facility for advanced management.
A-C, Brain MR imaging (FLAIR sequence) obtained 1 day after the development of toxicity and transfer demonstrated focal areas of vasogenic edema in the frontal lobes (arrows), parietal region (curved arrows), and occipital poles (open arrows) bilaterally with a mild degree of severity. Frontal lobe signal intensity is linear along the superior frontal sulcus (arrows), disconnected from the parietal abnormality (curved arrows) consistent with PRES and judged grade 1.
D, MRA at the time of initial MR imaging demonstrates extensive vasospasm of first-, second-, and third-order branches in the anterior cerebral artery (arrowheads), middle cerebral artery (arrows), and posterior communicating artery (short arrows) vessels bilaterally. A “node”-like appearance is seen at many branch points of the main parent vessels typical of spasm (black arrows 4G). Similar findings were also present in the posterior circulation.
E-F, Axial FLAIR image obtained on follow-up imaging study 11 days after the initial study demonstrates reversal of the vasogenic edema in all regions.
G, Repeat MRA obtained 11 days after the initial study demonstrates resolution of the extensive vasospasm with a near-normal appearance of all vessels (arrows).
Imaging features in patients with infection, sepsis, and shock
Vasogenic edema grade in Group 1 (Normotensive) and Group 2 (Severely Hypertensive) Patients
Follow-up imaging in 18 patients demonstrated reversal (12 patients) or partial/substantial reversal (6 patients) of the PRES imaging appearance. In 5 patients, follow-up imaging was not performed but the patients’ symptoms (altered mentation, headache, vision change) resolved completely. Two patients died of sepsis-related complications before repeat imaging was obtained.
Vasogenic Edema
Extent of brain edema was compared between patients from groups 1 and 2, and the results are summarized in Table 4. Vasogenic edema was judged greater in the “normotensive” patients (group 1: average 3.3) with less edema present in the “severely hypertensive” patients (group 2: average 2.07). This difference was statistically significant (Student t test: P = .01; Wilcoxon score: P = .03). Patients in group 1 demonstrated a greater range in the degree of brain edema; 4 of 11 (36%) demonstrated grade 4 or 5 edema (Figs 1–5). In contrast, patients in group 2 generally demonstrated either a mild or a moderate degree of vasogenic edema.
Vascular Features
MRA was available at the initial MR assessment of neurotoxicity in 10 of 25 patients with infection, sepsis, or shock (group 2 severe hypertension at toxicity: 6 patients; group 1 normotensive at toxicity: 4 patients). All 6 patients in group 2 demonstrated vessel irregularity at toxicity consistent with vasospasm (Fig 6). In 2 patients, initial MRA at toxicity demonstrated vasospasm, and follow-up MRA demonstrated reversal of spasm with vessel caliber and shape normalization.
In the patients in group 1 (normotensive), MRA at toxicity demonstrated vessel “pruning” with reduced second- and third-order branch visualization in 2 patients and vessel pruning with reversible spasm on follow-up MRA (Fig 5) in 1 patient. In 1 normotensive patient, MRA appeared normal.
Discussion
The imaging findings of “PRES” and associated neurotoxicity are recognized.1–15 This imaging pattern is typically seen in patients who develop eclampsia or cyclosporine/FK-506 neurotoxicity after transplantation, but other associations have been reported, including autoimmune disease (such as SLE or Wegener granulomatosis) and hypertension. These patients typically present with several days of headache progressing to visual disturbance and/or grand mal seizure.18 Unstable blood pressure frequently accompanies toxicity, but significant hypertension may be absent (25%–30% of patients).2,19
The cause of PRES is controversial and unproven. Neurotoxicity and PRES are associated with eclampsia, cyclosporine/FK-506 toxicity (typically after allo-BMT or solid organ transplantation), and systemic chemotherapy.1–11 PRES is also seen in patients with autoimmune disease, medical-renal disease, and severe hypertension.12–15 Isolated studies have suggested an association with hypomagnesemia, hypercholesterolemia, and human leukocyte antigen mismatch in allo-BMT.20
Potential biologic mechanisms for development of PRES have included factors that induce endothelial injury, such as the immune suppressive drugs cyclosporine and FK-506, endothelial activation/injury in eclampsia, endothelial injury from pretransplantation conditioning regimens, or graft-versus-host effects.2,21,22 Hypertension with forced hyperperfusion has also remained popular.3,23,24
Our results demonstrate 4 important observations in PRES including: (1) association with infection/sepsis/shock, (2) association with gram-positive organisms, (3) inverse effect of severe hypertension on the extent of vasogenic edema, and (4) high frequency of associated cerebral vasospasm.
PRES and Its Association with Infection, Sepsis, and Shock
The first unexpected observation in our study is that PRES seemed to occur in patients with infection, sepsis, and shock. In 18 of 25 patients, PRES occurred within 2 weeks of bacteremia or organ/tissue infection (abscess, pneumonia, wound infection), and in 3 patients, PRES developed within 30 days of abscess drainage or bacteremia. In 2 additional patients, neurotoxicity occurred within 2 weeks of severe hemorrhagic shock. If one eliminates patients with transplantation or eclampsia, significant infection, sepsis, or shock was present in 25 of 49 patients (51%) in the overall study group. We believe this is the first report to suggest that PRES may be associated with severe infection, sepsis, or shock.
Response to infection is an extremely complex process. At the fundamental biologic level, several systemic events occur simultaneously, including anti-infective, acute inflammatory, metabolic, pro-coagulant, and thermoregulatory changes.17,25–28 When infection is present, the role of the immune system is to 1) contain the infection, 2) target or traffic the inflammatory response to the infected region, and 3) prevent the inflammatory response from becoming systemic.17 If infection becomes overwhelming or if the immune response is inadequate, a septic clinical state can develop. The septic response to infection is known to occur without or with bacteremia (demonstrating identical morbidity and mortality), and cell-wall antigens of the infective organisms (endotoxin, exotoxin) are considered likely mediators.17,25–28 Both endotoxin (lipopolysaccharide [LPS] from gram-negative cell wall) and exotoxins/enterotoxins (peptidoglycan and lipoteichoic acid from gram-positive cell wall) are well known to be powerful stimulants of systemic inflammatory response, leukocytes, and endothelial interaction.17,28 It is noteworthy that no obvious source of infection can be identified in 30%–50% of cases that demonstrate a septic clinical presentation.17
Endothelial activation/injury is considered central to the development of the primary infection and secondary septic response.17,29–32 This process (which is mediated by inflammatory cytokine release [tumor necrosis factor (TNF)α, interleukin (IL)-1β, and other cytokines]) leads to up-regulation of endothelial surface antigens (P-selectin, E-selectin, ICAM-1) with increased white cell adherence, microcirculatory dysfunction, and altered vascular tone, vascular permeability, and coagulation.17,28,31,33,34 Microcirculatory dysfunction develops in part because of leukocyte adherence/trafficking with reduced local tissue blood flow at the capillary/venule level.34 An alteration in vascular tone develops secondary to competing vasoconstrictive (platelet degranulation with thromboxane release, endothelin-1, angiotensin, vasopressin, and central sympathetic stimulation) and vasodilatory (nitric oxide, prostacyclin) effects.17,33 Significant vascular instability has been documented in 50% of septic patients within 28 days of the infection.26
The potent vasoconstrictor endothelin-1 is released at its highest levels in sepsis, and morbidity/mortality in sepsis has been shown to parallel plasma endothelin-1 concentration.35–37 The inflammatory cytokines TNF-α and IL-1 up-regulate endothelin-1 mRNA production and stimulate its release from endothelial cells.38–40 Endotoxin also promotes the release of endothelin.41 Perhaps intermittent episodes of hypertension occur in sepsis but are difficult to recognize because of confounding factors (pain, intubation) and simple management with antihypertensive agents.
The clinical features of our patients parallel the general observations on sepsis with: 1) primary infection most commonly in the lungs, abdomen, wound or urinary tract, 2) blood culture-positive and blood culture-negative cases identified and 3) vascular instability and PRES developing within 14–30 days of severe infection. Endothelin-1 could contribute to the development of PRES in I/S/S.
Multiple Organ Dysfunction
The decision to group the patients with infection, sepsis, and hemorrhagic shock together is not arbitrary or for mere convenience. It is recognized that the systemic effects present in these conditions are similar and that a final common course/end point can occur with multiple organ system dysfunction and/or failure (MODS) including: coagulation, pulmonary, hepatic, renal, cardiovascular, and neurologic.30,42–44 This response to I/S/S seems to represent the effects of systemic toxicity similar to the recently described “Systemic Inflammatory Response Syndrome” (SIRS) or MODS.27,42–47 Cytokine response (TNF-α, IL-1) is believed to play a critical role in the development of this effect.42–45,48
In 18 of our patients with I/S/S, additional multiple organ system dysfunction (separate from neurologic and cardiovascular) was identified coincident with neurotoxicity and PRES similar to patterns observed in patients with MODS.
Gram-Positive Sepsis
The second important observation in these patients is the unexpectedly high incidence of gram-positive infection. In 16 of 19 culture-positive patients (84%), gram-positive organisms were identified in the primary infection site, blood cultures, or both. Gram-positive organisms are becoming more frequently identified in association with bacteremia and sepsis from both community-acquired and nosocomial sources with Staphylococcus aureus, coagulase-negative staphylococci, and enterococci accounting for 30%–50% of cases.49–51
The mechanisms involved in gram-positive sepsis are different from gram-negative sepsis with cell surface antigen (exotoxins: peptidoglycan and lipoteichoic acid) and superantigen-related T-cell stimulation of cytokine release compared with the more limited traditional T cell trigger of inflammation/cytokines as occurs with endotoxin/LPS from gram-negative organisms.17,52–56 Superantigens demonstrate a markedly greater interaction rate with the overall T cell population (5%–20% of T cells for superantigens versus 1 in 104-106 T cells for traditional antigen) with broader T cell stimulation and cytokine response.17,52 This broader, more generalized response to gram-positive organisms could underlie the onset and systemic manifestations in patients with PRES.
Although our study was not designed to assess the incidence of PRES in infection/sepsis/shock, an approximate reference point can be drawn from the predicted incidence of septic shock in patients with sepsis. The incidence of sepsis in the United States is estimated to be approximately 240 patients/100,000 persons per year; septic shock is estimated to occur in approximately 7%–8%.27,57 For our region, this would translate into 330 to 380 cases of septic shock per year distributed among 20 hospitals (approximately 115–190 gram-positive; 5.7–10 cases per year). The number of patients we identify with infection/sepsis and PRES (approximately 2.5–3 patients per year) is similar to the incidence of gram-positive septic shock for a single institution in our geographic region (excluding transplantation).27, 58 It is interesting that acute graft-versus-host disease (GVHD) seen after allo-BMT has been labeled a “distortion of the cellular response” to infection.57
PRES Imaging Appearance, MRA, and Hypertension
The third crucial and unexpected observation in our patients with I/S/S is that the extent of brain edema graded on imaging studies appears to be inversely related to blood pressure at toxicity. Normotensive patients (average MAP, 95 mm Hg) demonstrated the greatest degree of vasogenic edema, whereas severely hypertensive patients (average MAP, 137 mm Hg) demonstrated less brain edema, and this difference is statistically significant (P < .05). Fourth, in those patients for whom MRA was available, clear evidence of vasospasm or vessel “pruning” was observed. In the patients with severe hypertension, typical features of vasospasm were present and reversible where follow-up MRA was available for comparison. In normotensive patients, a combination of second- and third-order vessel “pruning” were noted. The reason behind these observations is not certain.
Although hypertension is frequently cited as the cause of PRES, blood pressure is not elevated in all cases. In 20%–30% of patients with cyclosporine toxicity or eclampsia, blood pressure is normal at toxicity.2,19 In preeclampsia/eclampsia, hypertension develops secondary to and along with complex systemic interactions, including endothelial activation/injury, platelet consumption, hemolysis, resultant vasospasm and vascular constriction, endothelial leakage, and organ hypoperfusion.59–61 Similar systemic effects are also present in other conditions, including transplantation and autoimmune disease.57,62–78 These issues suggest that a mechanism other than hypertension could be responsible for PRES.
Vessel “pruning” in our normotensive patients could reflect slow or sluggish cerebral blood flow, perhaps related to microcirculatory effects, such as enhanced platelet/white cell adherence/trafficking at the capillary/venular level.29,34 The effects of microcirculatory abnormality might be demonstrated by a flow-sensitive technique such as MR perfusion.
In the “severely” hypertensive patients, recognizable vasospasm could reflect involvement of larger cerebral vessels in the “vasculopathy” process. Alternatively, better vessel visualization (lack of pruning), observed vasospasm, and reduced vasogenic edema in these patients could suggest that hypertension is acting in a positive fashion to improve cerebral blood flow at some point in the toxicity process. Therefore, the role of hypertension and its contribution to the development of PRES is unclear.
Major Conditions Associated with PRES and Summation of Causes in Transplantation
Most the patients in our overall study population (100 of 106 patients [94%]) developed neurotoxicity and PRES in the setting of a complex systemic condition/illness, including infection, sepsis, shock: 25, chemotherapy: 4, autoimmune disease: 11, CsA/FK-506 (allo-BMT, solid organ transplant, marrow disorders): 49, and eclampsia: 11. The pathophysiologic mechanisms underlying these conditions are similar and include: immune system dysfunction, endothelial activation/injury, and/or a complex cytokine response.57,59–66 In eclampsia, immune challenge by the fetus and placenta is well recognized and complex immune reaction to fetal antigen is noted, including T cell, endothelial, and coagulation system activation.59–61 A similar response is noted after transplantation, in particular after allo-BMT with a T cell and cytokine response that has been likened to the response to viral or bacterial infection.57 In autoimmune diseases, a complex T cell immune and antibody response is felt responsible for most observed abnormality.62–66
After transplantation, patients are intrinsically exposed to most of the major risk factors we identify associated with PRES. Opportunistic infection is well recognized after transplantation, in particular immediately after transplant.67–69 The effects of cyclosporine/FK-506 and complications of transplantation often coexist, including endothelial injury with systemic effects.70–72 In allo-BMT, a direct effect of chemotherapy may also be present related to local endothelial turnover.21,22 A tissue injury response is also noted within the first 7–10 days after preconditioning regimens accompanied by a brief but recognizable cytokine response (IL-2, TNFα, IL-1).73–77
A markedly altered immune state is present after transplantation including GVHD and graft rejection. Acute GVHD after allo-BMT is generally related to either a T cell-mediated response of the graft to the host (in particular to host endothelium) or a response to the preconditioning regimens.17,18,31,32 Cytokines may play a prominent role in this effect (IL-2, IL-6, TNFα, IL-1).22,57,73–76 Graft rejection in solid organ transplant is related to the development of both T cell activation and anti-vascular/anti-endothelial antibodies.77,78
Transplants therefore may be experiencing a summation of the fundamental factors that are associated with the development of neurotoxicity and PRES, including infection (opportunistic), immune-related effects, chemotherapy-related effects, and the effects of cyclosporine or FK-506.
Conclusion
PRES occurred after severe infection, sepsis, or shock in 25 (23.6%) of 106 patients with other “associations,” including autoimmune disease, postchemotherapy, cyclosporine/FK-506, and eclampsia. In most patients with available cultures (84%), gram-positive organisms, particularly gram-positive cocci, were identified in organ/tissue culture, blood culture, or both. Normotensive and severely hypertensive patients were present in the I/S/S group. The extent of brain edema graded on CT/MR imaging studies was greater in the normal MAP group and less in patients who were severely hypertensive. Vasospasm was noted at MRA in patients who were severely hypertensive. A combination of spasm and vessel “pruning” was seen in MRA in the normal MAP group, perhaps related to reduced branch visualization secondary to diminished cerebral blood flow.
References
- Received November 22, 2005.
- Accepted after revision February 1, 2006.
- Copyright © American Society of Neuroradiology