Abstract
BACKGROUND AND PURPOSE: Intracerebral hemorrhages after embolization of arteriovenous malformations (AVMs) are the most dreaded complications of this well-established therapy. Apart from the known risk factors, our center noticed a high incidence of complications during postinterventional monitoring in medical intensive care units (ICUs) and stroke units.
MATERIALS AND METHODS: We report 125 consecutive interventions performed on 66 patients by using flow-dependent microcatheters and n-butyl cyanoacrylate as the embolic agent. Postinterventional intensive care monitoring was performed in an interdisciplinary operative ICU, a stroke unit, or a medical ICU. Patients were compared with regard to bleeding complications, AVM morphology, embolization result, postinterventional monitoring, and demographic factors.
RESULTS: Intracerebral hemorrhages occurred in 7 patients. Significant differences in outcome were found between 66 patients monitored in the interdisciplinary operative ICU from medical ICU or stroke unit. This was also true when adjusted for age and extent of AVM reduction by using exact logistic regression. A partial AVM reduction of >60% was a considerable risk factor for hemorrhage (odds ratio [OR] = 18.8; 95% confidence interval [CI] [1.341, not available]. Age was also an essential risk factor. An age difference of 10 years leads to an OR of 2.545 (95% CI [1.56, 7.35]).
DISCUSSION:A considerable AVM reduction in one session appears to increase the risk of hemorrhage technically. This suggests a distribution of the interventions in many partial steps.
There are basically 3 options for treating arteriovenous malformations (AVMs): neurosurgical interventions, stereotactic irradiation, and endovascular embolization. Endovascular therapy has prevailed as the first-choice strategy at most centers. Surgery and irradiation are more commonly used for treating any remnants that may have escaped embolization. The efficiency and procedural risk of all 3 methods have been thoroughly examined.1–7 The literature, however, has directed less attention to another frequent complication, postinterventional bleeding in the first few days after embolization. In particular, various causes are discussed, including venous embolization, special morphologic features, and hemodynamic changes,1 though it remains unclear precisely what factor puts patients at an above-normal risk for this postinterventional complication. Moreover, the literature discloses a wide unexplained variation of 1% to >10% in the incidence of such a complication, with larger centers reporting values of around 3%–4%.1–7 The primary goal of our study, however, was not identification of risk factors for postinterventional bleeding after AVM embolization. This was a by-product of processing the cases. Instead, our study was prompted by the inexplicable finding that conservative intensive care units (ICUs) such as the medical ICU and the stroke unit had a higher incidence of such complications than did the anesthesiologically managed surgical ICU.
Materials and Methods
The assessment included 125 consecutive interventions in 66 patients from January 1, 2000 to September 30, 2002. The patients were 26 women and 40 men ranging in age from 6 to 74 years, with a mean age of 38 years. All 125 procedures were performed in the same manner. All embolizations were carried out under insufflation anesthesia. Transfemoral access was achieved with a 6F valved introducer sheath. A 6F guiding catheter was inserted via the sheath with continuous heparinized high-pressure irrigation and placed in the internal carotid or the vertebral artery. This catheter was used to insert flow-dependent microcatheters with diameters of 1.2F, 1.5F, or 1.8F (Magic; Balt, Montmorency, France), depending on the flow and diameter of the AVM-supplying arteries. They were advanced intranidal in a wedged position, if possible, or as close as possible to the nidus. If necessary, this was also done with the aid of guidewires, but the tip of the wire never extended beyond that of the microcatheter. Each embolization was preceded by superselective angiograms, usually in 2 planes. Embolization itself was performed in all cases with n-butyl cyanoacrylate (Histoacryl; Braun, Melsungen, Germany). As many as 3 different vascular areas were embolized in one session. After completion of the embolization, a control angiography was generally performed in 2 planes. The patients were then monitored in an ICU, in most cases for 24 hours, and a strict upper systolic blood pressure limit of 140 mm Hg was maintained. Beyond the heparinized flush of the guiding catheter, no additional heparin was given, so no reversal of heparin effect was necessary. A postinterventional cranial CT scan is not routinely performed at our center. In cases where there was clinical suspicion of periprocedural or postinterventional intracranial hemorrhage or any other neurologic symptom, however, a CT scan was carried out immediately. Patients with postinterventional bleeding and those with uncomplicated interventions were compared with regard to the AVM morphology and embolization-induced morphologic changes, focusing on venous drainage, demographic factors, and postinterventional monitoring, particularly the blood pressure and coagulation course. The AVM size (mm) was measured in 3 dimensions and the product divided by 2 with regard to the eliptoid or round forms of AVMs.8,9
Statistics
Because our unit of analysis is always the patient and 2 treatments in a single patient are never considered independently in terms of statistics,10 we performed statistics only on the first embolization in every patient, with the knowledge that this describes the situation only partially. One patient bled after the second embolization, so she was not used in the calculation. We used the Fisher exact test for an initial group comparison. To take potential confounding factors such as age or the amount of reduction into account, an exact conditional logistic regression was performed. A significance level of P < .05 was chosen. All calculations were performed with the statistical package SAS 9.1 (SAS Institute, Cary, NC). To make the numbers comparable for the reader with regard to number of interventions, we give total results in parentheses whenever possible in the text as well as in Tables 1 and 2.
Comparison of AVM size, reduction in one session, and age of patients with and without a bleeding complication
Comparison of postinterventional monitoring of patients with and without a bleeding complication
Results
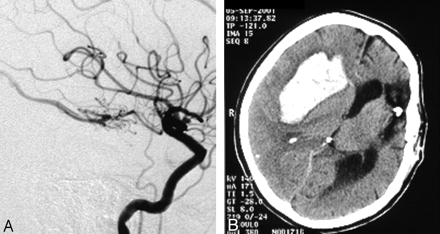
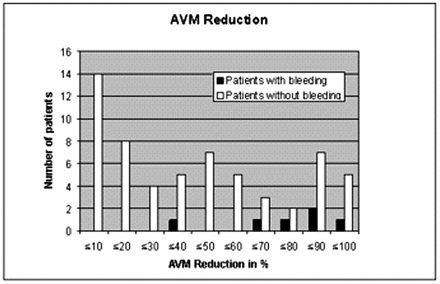
A total of 6 patients (7/125 interventions) with postinterventional bleeding were monitored during the observation period. Two typical examples are given in Figs 1 and 2. Most striking in this connection was the considerable impact of a large AVM reduction in one session. Five of the 6 patients with postinterventional bleeding achieved an AVM reduction of 60% or more in one session, whereas only 35% of the patients without hemorrhage achieved such an occlusion rate (Fig 3) at a time. On the basis of the logistic regression, an odds ratio of 18.8 (95% CI [1.341, not available]) for extension of AVM reduction indicates the impact of this matter. Another striking factor was the age of the patients. Half the hemorrhage patients were 50 years or older. Only 15% of all the patients were older than 50 years of age (Table 1, Fig 4). Therefore, an age difference of a decade implies an odds ratio of 2.545 (95% CI [1.56, 7.352]). Equally striking was the distribution of patients for postinterventional monitoring (Table 2). This corroborated our initial suspicion that had originally prompted us to process the data. Five of the 6 bleeding complications were seen in the stroke unit or medical ICU. Only one hemorrhage occurred in the interdisciplinary operative ICU. An important observation was the influence of the nidus size. All 6 bleeding complications were associated with a nidus volume of <3 mm, and none of the patients with a large nidus size had a bleeding complication. Unfortunately, for technical reasons, this covariate could not be included into the exact logistic regression model. Other frequently mentioned risk factors for bleeding complications did not prove to be particularly striking for the hemorrhage group. A relative exception, however, was accidental venous embolization, which was observed in a total of 32 patients (49/125). Four (5/7) of these patients then had intracerebral bleeding in the further course. Stenoses of the draining veins could be detected in only 4 patients, one of whom had a hemorrhage after embolization. Arteriovenous fistulas were found in 23 patients. Here too, bleeding occurred in only one case. The singular draining vein often regarded as a hemorrhagic risk factor was found in only one patient, who also had a bleeding complication. The intranidal or afferent-artery aneurysms often mentioned in connection with an increased risk of hemorrhage were seen in 24 of our patients, 2 of whom had bleeding complications in their postinterventional courses. No striking distribution patterns were found for other factors like the localization or morphology of the AVM, proliferative angiopathy or topography of the venous drainage or Spetzler-Martin grading. The only striking finding relating to the number of afferent arteries was the fact that all bleeding complications occurred in patients with a maximum of only 3 afferent arteries. This, however, also applied to about 60% of the total patient population.
Female patient with small AVM completely occluded in one session. Bleeding 19 hours after embolization.
Venous passage of embolic agent. Bleeding 24 hours after embolization.
Differences in the distribution of AVM reduction in one session in patients (interventions) with and without (w/o) a bleeding complication.
Different age distribution of patients with and without postinterventional bleeding.
Discussion
Our retrospective study was prompted by the increased incidence of postinterventional bleeding complications in the stroke unit and medical ICU, though subclinical hemorrhages were possibly missed, because we do not perform CT scans routinely after embolization therapy and procedures are carried out under general anesthesia in our institution. This was clearly demonstrated by the assessment with 5 of 6 hemorrhages in the stroke unit and the medical ICU as opposed to only one bleeding complication in the anesthesiologically managed interdisciplinary operative ICU. One simple explanation could be an unfortunate random distribution, which would be statistically conceivable with such small numbers; however, we initially suspected a systematic error such as faulty systemic heparinization or nonadherence to blood pressure limits. In the postinterventional care of AVM patients, we usually attach importance to strictly maintaining an upper systolic blood pressure limit of 140 mm Hg7 and dispense with any heparin application precisely because of the relatively frequent bleeding complications. The unfortunately incomplete documentation evidenced a few violations of this regimen, but never in connection with the bleeding complications encountered. A search for systematic differences between the ICUs involved reveals a marked difference in the patient population. The “average” patient in a stroke unit or a medical ICU tends to be older, has had a stroke or myocardial infarction, and is usually treated with systemic heparinization or even fibrinolysis and is kept in a more hypertensive state. In contrast, “typical” postoperative patients tend to be younger, likewise receive thrombosis prophylaxis, and are usually kept strictly below a defined systolic blood pressure value. Thus, postinterventional AVM patients tend to resemble the “typical” operative ICU patient and not those in a stroke unit or medical ICU. Our suspicion was—and is, despite the lack of evidence—that AVM patients in stroke units and medical ICUs received a treatment at the upper blood pressure limit, whereas in the surgical ICU patients are kept closer to the lower blood pressure limit. Moreover, we have noted that the stroke unit and medical ICU, as opposed to the operative ICU, differ markedly in the reaction times to factors like the increase of blood pressure above the upper limit and the aggressiveness of therapeutic measures taken against such an event, possibly reflecting the experience with hemorrhage in different parts of the body. The department-specific differences are surely not causal for a bleeding complication in most cases but may increase the risk of hemorrhage. In patients at high risk for hemorrhage—ie, older, small malformations, high partial reductions, and venous embolization—the demand for strict adherence to blood pressure limits is a consequence not only documented in writing in the angiography protocol but has also been noted in the anesthesia protocol and orally communicated to the attending staff physician, particularly when a limited capacity necessitates postinterventional monitoring in the stroke unit or medical ICU rather than the operative ICU. No heparinization is administered. Noteworthy in this connection is also the “normal pressure breakthrough” phenomenon first described by Spetzler et al,11 in which a postoperative or postembolization reduction of flow through the AVM in conjunction with a pre-existing steal phenomenon can lead to increased perfusion in the surrounding brain tissue with subsequent edema and bleeding. Such an effect would be markedly enhanced by systemic heparinization and a hypertensive blood pressure status. Venous passage of the embolic agent with resultant venous obstruction or even thrombosis is a long-known and thus by no means surprising risk factor for postinterventional bleeding.3,7,12 Somewhat more surprising, however, is the frequent and usually complication-free occurrence of such a venous embolization. Because AVMs are now intranidally embolized whenever possible, other study groups also describe frequent venous passage of the embolic agent.2,3,7,12 In principle, this surely represents a potential bleeding risk, but in practice it is usually of secondary importance if venous drainage of the AVM is still adequately ensured via other veins. Moreover, even venous thrombosis is described as a spontaneous recovery in individual cases.13 Striking in our series is the occurrence of bleeding complications particularly after a considerable AVM reduction in one session. This at first seems surprising but is in agreement with the results published by Picard et al.2 This study group also noticed an increased complication rate after a considerable partial reduction in a much larger patient population than ours. This could possibly be explained on the basis of an often-described pressure increase in the afferent arteries after intranidal embolization by using fluid embolic agents with the potential risk of an arterial or intranidal vascular rupture.3,14–17 Such an increased pressure, however, may also be caused by venous thrombosis that can develop either from partial venous embolization or from venous stasis after a considerable flow reduction. Whether this pressure increase is age-related has not yet been clarified but would be conceivable, because long-standing malformations can lead to autoregulation disorders. This would explain our observation of an increased risk for older patients. An enhanced bleeding risk was also seen in malformations <3 mm. This, too, is not surprising, because small AVMs are generally associated with an increased bleeding risk. Moreover, the relative obliteration rate in one session is of course markedly higher for a small AVM, and a high flow reduction can be achieved in one session even without extensive embolization, which in turn increases the risk of a reactive pressure increase, as described above.
Conclusion
Altogether, we found that bleeding complications after interventional AVM treatment were significantly associated with an older age of patients as well as high percentage of partial embolization and postinterventional follow-up. Postinterventional monitoring appears to be better ensured in an anesthesiologically managed operative ICU than in medical ICUs or a stroke unit. This led us to divide the treatment of an AVM into many partial embolizations with only small flow reductions in single sessions.
References
- Received March 3, 2005.
- Accepted after revision July 18, 2005.
- Copyright © American Society of Neuroradiology