Abstract
BACKGROUND AND PURPOSE: Diffusion alterations have been identified in the corpus callosum and frontal white matter of patients infected with human immunodeficiency virus (HIV), though the relevance of these findings to cognitive deterioration has not yet been determined. This study tested the hypothesis that diffusion tensor imaging can detect tissue status alterations in these regions in cognitively impaired patients infected with HIV and the acquired measurements correlate with the severity of cognitive impairment.
METHODS: Fractional anisotropy (FA) and mean diffusivity (MD) were determined for corpus callosum (genu and splenium) and frontal white matter (FWM). The DTI measurements were compared in 11 HIV and 11 control participants. Patterns of relationship were examined with cognitive status measures from concurrent neurologic and neuropsychologic evaluations.
RESULTS: FA values for the splenium were significantly reduced in the patients infected with HIV and correlated with dementia severity and deficits in motor speed. MD values for the splenium were significantly increased in the patients infected with HIV and correlated with deficits in motor speed. FA measurements were also significantly correlated with performance on visual memory (genu), visuoconstruction (FWM), and verbal memory (FWM) tasks.
CONCLUSION: Diffusion abnormalities were identified in the splenium of the corpus callosum in patients infected with HIV, and these alterations were associated with dementia severity and motor speed losses. In vivo assessment of callosal integrity by using quantitative neuroimaging may have potential utility as a marker of brain injury in patients infected with HIV.
Patients infected with human immunodeficiency virus (HIV) may eventually present evidence of neurologic involvement, including cognitive deterioration. Autopsy studies of HIV-dementia (HIV-D) patients indicate prominent injury, including increased numbers of microglia, macrophages, astrocytes, and multinucleated giant cells, in basal ganglia and deep white matter.1,2 Diffusion tensor imaging (DTI) is sensitive to the microstructural integrity of tissue and can be used to obtain noninvasive measurements of the mean diffusivity (MD) and the fractional anisotropy (FA) of water molecules in brain regions of interest.3 DTI measurements acquired in subcortical regions correlate with cognitive status measures in patients infected with HIV.4 Diffusion abnormalities involving the frontal white matter (FWM) and the corpus callosum have also been observed in patients infected with HIV,5,6 though the relationship to cognitive impairment has not yet been determined. In this investigation, DTI was used to derive tissue status measurements in corpus callosum (genu and splenium) and in FWM of cognitively impaired patients infected with HIV to determine the significance of injury in these regions to dementia severity and deficits in attention, memory, constructional abilities, and motor speed. This study tested the hypothesis that DTI can detect tissue status alterations in these regions in cognitively impaired patients infected with HIV and the acquired measurements correlate with the severity of cognitive impairment.
Methods
Participants
Seropositive subjects (mean age, 49.4 ± 7.3 years; 9 men and 2 women) included 11 well-characterized patients participating in a longitudinal investigation of the natural history of neurologic impairment in advanced HIV infection. Six of the 11 patients infected with HIV received Memorial Sloan Kettering Rating Scale (MSK)7 ratings of 0.5, 4 of the patients received ratings of 1, and a single patient received an MSK rating of 2. Control subjects included 11 healthy volunteers, without history of neurologic illness (mean age, 42.4 ± 11.2 years; 9 men and 2 women). There were no significant differences between the groups in age or education. Self-reported seropositivity was confirmed by enzyme-linked immunosorbant assay and Western blot. CD4 counts for the patients infected with HIV ranged from 24 to 427; plasma viral load ranged from undetectable to 154,938 copies/mL. The patients infected with HIV in this study were medically stable and had been receiving antiretroviral treatment for an average duration of approximately 5 years. One patient’s therapy was temporarily suspended at the time of the scan. Study exclusion criteria included chronic neurologic disorders, current or past opportunistic central nervous system (CNS) infection, psychosis at study entry, or MR contraindications. The investigation was conducted with approval from our institutional review board.
Clinical assessments of the patient with HIV included the macroneurologic examination created by the AIDS Clinical Trial Group and the motor portion of the Unified Parkinson Disease Rating Scale, used to assess extrapyramidal signs. The neuropsychologic examination evaluated working memory, verbal memory, visual memory, constructional ability, psychomotor, motor speed, and frontal/executive functions. The cognitive domain measures were based on composites of standardized scores of individual subtests included in the battery. Each of the cognitive domain measures was scaled so that lower scores reflect poorer performance. Dementia severity was determined on the basis of criteria defined by MSK7 and by the American Academy of Neurology (AAN).8 The Karnofsky Performance Scale was also used to assess functional status.9 Both AAN and MSK criteria have been operationalized for uniform staging across multiple research sites.10 The operationalized MSK scoring takes into account the presence of CNS abnormalities on examination, the results of the neuropsychologic testing and the degree of impairment in work, self-care, and mobility status reported by the patient. A reported deficit in at least one of the 8 instrumental activities of daily living is required to meet the minimal functional criterion for MSK staging. The AAN ratings were determined on the basis of the degree of impairment on the neuropsychologic tests by using an extensively validated computer algorithm that has been used in many prior studies of HIV-D10 The derivation of the cognitive domain measures and the operational definitions of the dementia severity ratings have been described in extensive detail in previous reports.10,11
MR Imaging and Image Processing
Imaging studies were performed on a 1.5-T twin-speed MR unit (GE, Milwaukee, Wis) equipped with the zoom gradient. A quadrature birdcage head coil was used for radio-frequency transmission and signal intensity reception. DTI was performed with an echo-planar sequence and a bandwidth of ±125 kHz. A b = 0 reference image and 6 diffusion-weighted images with b-values of 1000 seconds/mm2 were acquired of each section. Diffusion gradients were applied along 6 directions. The entire brain was imaged, inferior to superior, from the base of the cerebellum to the top of the skull, by using 22 contiguous 7-mm axial sections with the following parameters: field of view, 24 cm; matrix, 128 × 128; 7000/4 (retention time [TR]/number of excitations).
Quantitative image analysis was performed off-line. A custom software package, DPTools,12 was used to calculate the FA and MD and to place the regions of interest. The b = 0 reference image was used for region of interest placement to achieve better anatomic visibility. The regions of interest were automatically projected onto the FA and MD maps to acquire the DTI values. Uniform-sized regions of interest (43 mm2) were placed by using a consistent strategy for all subjects, by an operator who was blinded to group status (HIV or control). Regions of interest for the genu and splenium of corpus callosum were identified along the midline at the plane of the interventricular foramen. Regions of interest for FWM of each hemisphere were identified anterior to the bilateral ventricle frontal horns (Fig 1). The DTI values for left and right hemispheres were averaged to generate the FA and MD measurements for FWM.
Uniform-sized regions of interest were placed on anatomic T2-weighted image (left) and then projected to FA (middle) and MD (right) maps.
Routine visual inspection of the images indicated the atrophic changes that have been described in many previous MR studies of patients infected with HIV13.
Statistical Analyses
Primary dependent measures included the DTI measures (MD and FA) acquired in corpus callosum (genu and splenium) and in FWM. These measurements were compared in HIV and control subjects. Dementia severity and neuropsychologic measures of specific cognitive functions were also examined. All statistical tests were 2-tailed, using a significance level of 0.05, and were executed in SPSS (release 12.0; SPSS, Inc., Chicago, Ill).
Results
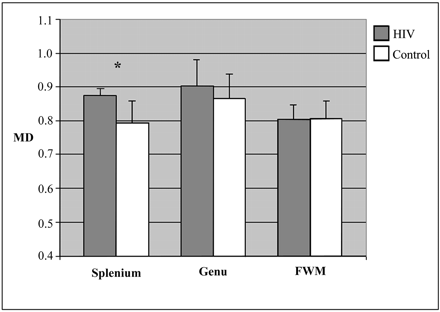
Between-group differences were evaluated by using analysis of variance models with age entered as a covariate. In the splenium, FA measures were significantly reduced (P = .023), and MD measures were significantly increased in the HIV subjects (P = .050). There were no significant between-group differences for the FA or MD measures in the genu and FWM, although MD values for the genu were higher in the patients infected with HIV and this difference was nearly significant (P = .08). Means and SDs for the localized DTI measures are presented in Figs 2 and 3.
Means and SDs of functional anisotropy (FA) measurements. *P < .05.
Means and SD of mean diffusivity (MD) measurements. MD is expressed in units of 10−4 mm2/sec *P < .05.
Further analyses examined associations between the DTI measures and the dementia severity ratings (AAN, MSK, and Karnofsky). Reduced FA measures for the splenium were significantly associated with dementia severity as indicated by MSK (ρ = −.53; P = .012), AAN (ρ = −.52; P = .015), and Karnofsky (ρ = 0.44; P = .042) ratings (Table 1). Table 2 presents correlations between the localized FA measures and specific cognitive domains. FA measures in the splenium were significantly correlated with motor speed deficits (r = 0.47; P = .03). Increased MD in the splenium was also significantly correlated with motor speed deficits (r = −.60; P = .003). A significant correlation was also identified between FA for genu and visual memory deficits (r = 0.44; P = .043). FA measurements for FWM were significantly correlated with deficits in visuoconstruction (r = 0.46; P = .031) and inversely correlated with verbal memory (r = −.49; P = .022). No other significant relationships were identified for the MD measures, although the correlation of MD for FWM and frontal/executive function was nearly significant (P = .07). Clinical markers—including CD4 counts, plasma viral load, and body mass index—were not significantly associated with the DTI measures in the HIV subjects.
Correlations between fractional anisotropy and dementia severity ratings
Correlations between fractional anisotropy and cognitive domains
Discussion
HIV infection mediates different degrees of brain injury with progression to cognitive impairment and dementia (HIV-D) in some patients. Imaging techniques have been exploited to determine neuropathologic substrates underlying HIV-D (reviewed by Tucker et al14). Many prominent features of HIV encephalitis (HIVE), the pathologic correlate of HIV-D, however, cannot be detected by conventional T1- or T2-weighted MR imaging.15 Determination of mechanisms underlying injury and the relationship to specific cognitive sequelae in patients infected with HIV has been hindered by the difficulty monitoring ongoing changes in brain tissue. Strategies such as DTI have been used in efforts to quantify brain injury that may not be apparent on conventional images.4–6,16,17
In this investigation, anisotropy measurements for the splenium were significantly reduced and correlated with the severity of dementia in patients infected with HIV. The MD for this region was significantly increased in patients infected with HIV. DTI alterations (FA and MD) in the splenium were also significantly correlated with motor speed. Psychomotor slowing is predictive of dementia progression18,19 and HIVE at autopsy.20 Reduced anisotropy in the splenium has been reported in patients infected with HIV who have high viral loads,6,21 whereas changes in this region have not been found in patients infected with HIV who have undetectable viral loads6 or in neurologically asymptomatic patients infected with HIV.5 Because cognitive decline generally presents in advanced stages of HIV infection, these findings suggest the possibility that DTI measurements acquired in the splenium may demonstrate meaningful variation associated with neurologic progression in HIV infection.
It is well established that HIV-D is associated with injury to basal ganglia and deep white matter.1,2 It has been suggested that injury in the corpus callosum of HIV-D patients has not been adequately recognized.22 Early pre–highly active antiretroviral therapy (HAART) structural imaging studies reported high-signal-intensity lesions in the splenium in cognitively impaired patients infected with HIV23 and an association between abnormalities in this region and dementia in patients infected with HIV.24 Imaging abnormalities have also been reported in the corpus callosum among patients infected with HIV in the HAART treatment era,6 including findings of callosal white matter thinning in cognitively asymptomatic patients infected with HIV.25
Autopsy studies of patients infected with HIV have observed neuropathologic findings in the corpus callosum.22 Some neuropathologic evidence suggests preferential invasion of brain white matter regions, including corpus callosum, by infected macrophages and multinucleated giant cells.26 Animal experiments by using iron oxide for tracking macrophages have found distribution along the injection site (in basal ganglia), the corpus callosum, the ventricular system, and into other brain regions when examined across a 2-week postinjection period.27 Animal models of neuroAIDS demonstrate that significant changes in FA can be detected in the splenium within 2 weeks of initial infection28 and have identified an association between axonal damage in corpus callosum and motor deficits.29
The functional and topographic organization of corpus callosum has not been completely characterized for the human brain. Formulations of callosal function emphasize multiple routes for transfer of information between hemispheres. The corpus callosum plays a role in visuomotor integration and may interact in important ways with subcortical structures, notably basal ganglia, in response initiation.30 Injury may be reflected in slowed response initiation and longer reaction times on tasks involving hemispheric transfer or integration between regions. Changes in the integrity of this structure may be associated with less efficient compensatory mechanisms. Patients with multiple sclerosis who have evidence of callosal involvement show evidence of deficits in interhemispheric coordination of motor activity.31 Otherwise asymptomatic callosal agenesis subjects are consistently slower on simple reaction time tasks and clinical disorders associated with this condition are often characterized by motor impairment.32 Motor losses are considered among the most sensitive indicators of early cognitive decline in patients infected with HIV33 and have been used to monitor treatment response.34 In addition to interhemispheric transfer, formulations of callosal function emphasize potential involvement in mediating and optimizing hemispheric coactivation and minimizing arousal asymmetries to enable coherent, conjoint activation of the 2 hemispheres.35 According to this view, specific impairments (eg, visuoconstruction, slowed reaction times) due to agenesis, surgical section, or insult to the corpus callosum may reflect loss of transcallosal enhancement of cortical activity and resulting loss of efficiency in cognitive performance.32
The relationships observed between anisotropy reductions in the studied regions (splenium, genu, and FWM) and cognitive deficits may have a basis in axonal injury. Axonal injury has been associated with neurologic outcome in white matter diseases and in CNS infections.36 Autopsy examinations using sensitive quantitative markers for detecting early injury (β-amyloid precursor protein [β-APP] immunoreactivity) indicate widespread axonal damage in tissue samples from HIVE patients.37 It has been suggested on the basis of β-APP findings that cognitive deficits in patients infected with HIV may be due to progressively severe axonal injury and factors such as swelling of injured axons, disturbed axonal transport, and axonal loss.38 These white matter alterations may be reflected in the measured anisotropy.39,40 Multifocal distributed neural networks, interconnected by white matter pathways, are regarded as critical to an understanding of higher-order cognitive function and dysfunction.41 The corpus callosum represents the largest commissural fiber pathway in the human brain, and this structure is involved in many functional networks.41 The observed relationships between diffusion alterations and cognitive deficits may reflect conduction deficits as a result of localized injury or loss of integrity in the networks in which the studied white matter regions (splenium, genu, and FWM) participate.
In this investigation, there were no significant differences for DTI measures for FWM in the patients infected with HIV; anisotropy measures for this region were significantly correlated with visuoconstruction and inversely related to verbal memory. DTI abnormalities have been reported in the FWM of cognitively asymptomatic patients infected with HIV,5 and MR spectroscopy studies indicate that this region may be subject to early injury in patients infected with HIV.42 The intrinsic anisotropy in frontal regions is lower than in corpus callosum. In addition, the presence of multiple fiber directions within each voxel may complicate determination and interpretation of anisotropy measurements acquired in FWM,43 as well as the magnitude and directionality of relationships with cognitive status measures.39 In contrast, fiber tracts in the genu and in the splenium follow a very well-organized right-left directionality.
Mental status alterations have been identified as a common clinical correlate of splenium MR abnormalities across a wide spectrum of diagnoses, including HIV.44 Studies of other CNS disorders characterized by cognitive decline have found evidence that DTI measurements acquired in the splenium may be sensitive to subtle or early changes not apparent on conventional images.45–47 The FA measurement error is lowest in regions with intrinsically high anisotropy.43 The splenium has higher intrinsic anisotropy than any other region of the brain, including the genu.48 It has been suggested that for this reason, early or subtle changes may be more markedly manifest in this region.46 Readily identifiable anatomic landmarks, such as the splenium, may also facilitate acquisition of reliable tissue status measurements across patients and across time within the same patient.
Findings from this DTI investigation of cognitively impaired patients in the HAART era indicate that anisotropy measurements of the splenium may represent a promising quantitative imaging biomarker in the setting of HIV infection. Whether the observed findings are due to the sensitivity of anisotropy in this region for detecting more diffuse or distal injury and/or localized neuropathologic changes in the splenium is not yet clear. Larger, longitudinal investigations will be necessary to establish the validity of these measurements as biomarkers of neurologic status in HIV infection. Studies examining response to HAART treatment in patients infected with HIV will be important to determine whether DTI abnormalities observed in the splenium and other regions reflect reversible or more advanced, irreversible injury.
Conclusion
The corpus callosum, particularly the splenium, may be a promising region of interest for monitoring changes associated with neurologic progression in HIV infection. DTI measures acquired in the splenium were significantly associated with dementia severity (FA) and with motor speed (FA and MD), a sensitive marker of early cognitive decline in patients infected with HIV.
Acknowledgments
We are grateful for the assistance of Linda Pierchala, Linda Reisberg, and Renee Ochs.
Footnotes
This study was supported in part by National Institute of Mental Health grant MH66705 and National Institute of Neurologic Disorders and Stroke grants NS36519 and NS049465.
Presented in part at the annual meeting of the International Society for Magnetic Resonance in Medicine, May 2005.
References
- Received April 14, 2005.
- Accepted after revision July 26, 2005.
- Copyright © American Society of Neuroradiology