Abstract
SUMMARY: Patients with Klippel-Trenaunay-Weber syndrome present with venous varices, cutaneous capillary malformations, and tissue hypertrophy, usually involving an extremity. A small but important subset also harbors arteriovenous malformations (AVMs) of the spine. We report 2 such cases, 1 with 3 concurrent spinal arteriovenous fistulas. These cases and our review of the literature emphasize the importance of screening the spine for AVMs. In addition, it is also important to investigate for the presence of multiple spinal AVMs.
Patients with Klippel-Trenaunay-Weber (KTW) syndrome present with venous varices, cutaneous capillary malformations, and tissue hypertrophy.1 Klippel and Trenaunay described the association of the 3 findings in 1900 and Parke Weber later added the important finding of arteriovenous fistulas (AVFs) to the syndrome complex.2 The exact cause of KTW syndrome is not well defined; some authors claim in utero insults as a possible cause.3 The clinical presentation of this syndrome is variable.4 There are many reports of other associated congenital anomalies with this syndrome. Anomalies of the fingers and toes and arteriovenous malformations (AVMs) are among these anomalies.1, 3, 5
In this report, we discuss 2 patients with KTW syndrome who presented with neurologic symptoms related to associated AVMs involving the spinal cord and spine. We also performed a review of the literature.
Case 1
A 37-year-old woman was referred for a second opinion for symptoms associated with a spinal arteriovenous shunt. She had been treated for limb hypertrophy as a child by closure of the physeal plates and had had surgical removal of venous malformations from the right lower extremity. At the current presentation, the patient showed subtle residual findings of KTW syndrome consisting of venous varices involving the right foot and a temperature difference in the feet (warmer on the right) but no evidence of cutaneous capillary malformation. She complained of increasing right lower extremity radicular pain and some perineal pain. The pain increased with activities increasing central venous pressure, such as performing sit-ups or crunches, or with prolonged standing. MR images of the lumbar and thoracic spine demonstrated multiple abnormal enlarged intradural flow-voids, especially at the level of the conus medullaris, with serpentine vascular structures extending cranially and caudally (Fig 1). There was no spinal cord signal intensity abnormality. Dynamic gadolinium-enhanced MR angiography (MRA) demonstrated an intradural vascular malformation, with the largest abnormal flow voids in the central spinal canal from T12 to L2 (Fig 1). A limited spinal angiogram performed at an outside institution demonstrated a type 4 (pial) fistula with moderate flow and variceal dilation of the recipient venous pouch at the level of the conus medullaris. A large radiculomedullary artery arising from the left lateral sacral artery provided good access to the recipient venous pouch. Additional supply was also seen from the anterior spinal artery (ASA). Because the frequency and intensity of the patient's symptoms was increasing, endovascular treatment was offered to the patient.
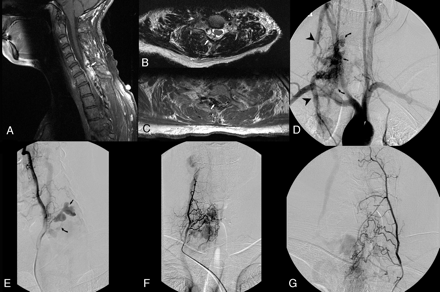
Case 1. Sagittal and axial T2-weighted images demonstrate the dilated venous recipient pouch for fistula 1 as well as dilated veins on the dorsal and ventral aspects of the cord (arrows, A and B). Contrast-enhanced MRA maximum intensity projections demonstrate the large venous recipient pouch of fistula 1 (arrowheads, C and D) and secondary draining veins (arrow, D). Left common iliac artery injection demonstrates a large radiculomedullary artery (curved arrow, E) arising from the left lateral sacral artery (arrow, E) and ascending toward the large venous recipient pouch supplying the fistula (not shown). Microcatheter injection at the level of fistula 1 opacifying the recipient venous pouch (arrow, F) and demonstrating secondary drainage into superiorly (arrowhead, F) and inferiorly (curved arrow, F) draining veins. Native and subtracted images from a right intercostal artery injection (T12) demonstrating the second (2) of the 3 pial fistulas (arrowhead, G) located at the level of inferior endplate of T11. Venous drainage is into a short venous channel (star), draining into a common channel (curved arrow) receiving venous drainage from both smaller fistulas (2 and 3). This common channel then drained into the venous pouch, which was the main recipient venous pouch for the largest fistula (1; arrow, G and H). Selective left T12 injection demonstrates pial fistula 3 (arrowhead, I), also at the level of the inferior endplate of T11.
After explaining the procedure and its potential risk of complications, informed consent was obtained for treatment under monitored anesthesia care. The right common femoral artery was accessed with a 6F arterial sheath, and a catheter was used for selective catheterizations and injections of the right T12 to L3 and left L1 to L3 arteries. Because type IV fistulas are generally single-hole fistulas, the spinal arteriogram was limited to the pedicles listed. The arteriogram revealed a type IVB arteriovenous fistula, fed by the ASA, and an enlarged radiculomedullary artery arising from the left lateral sacral artery. The venous varix draining the fistula was located primarily inferior to the conus medullaris and was drained by 3 veins, 2 draining inferiorly and the other draining superiorly. One of the 2 inferiorly draining veins was larger and extended to the sacral region and exited one of the sacral foramina. Endovascular treatment was begun by a guide sheath into the left internal iliac artery, through which a 5F catheter was placed into the lateral sacral artery. Using a triaxial system, a microcatheter was advanced through the radiculomedullary artery and into the venous varix draining the fistula. The varix was embolized using 8 detachable complex platinum coils (DCS; Cordis, Miami Lakes, Fla). Because of concern about creating significant mass effect on the conus by placement of additional coils, a standard mixture of n-butyl cyanoacrylate (n-BCA), ethiodized oil, and tantalum powder was injected to fill in the interstices of the coils and the venous varix. Control angiography done immediately after n-BCA embolization demonstrated one collateral artery arising from the lateral sacral artery, more proximal to the enlarged radiculomedullary artery with delayed filling of a very small part of the varix but without any flow beyond the varix. Control angiography of the left internal iliac artery, followed by a distal abdominal aortogram, demonstrated no filling of the varix or draining veins and no evidence of residual fistula at that time.
The patient had significant relief of her symptoms, with disappearance of the radicular symptoms, and a mild residual amount of perineal pain. Follow-up contrast-enhanced and time-resolved MRA of the spine performed on the day after embolization revealed a persistent arteriovenous shunt, despite clinical improvement. This MRA showed a persistent draining vein extending inferiorly from the AVM. Some venous structures also extended cranially from the AVM. The venous varix seen the day before was now less well-defined as a result of embolization. Given the MRA evidence for persistent arteriovenous shunt surgery, and despite continued symptomatic improvement, the decision was made to perform a follow-up spinal arteriogram 3 month later. On re-examination at that time, angiography revealed 2 additional separate smaller pial AVFs (type IVa). These 2 AVFs were located at the level of the inferior endplate of T11, on the dorsal surface of the cord, supplied by the posterior spinal arteries. Each of these 2 fistulas drained into short separate venous channels, which converged, in a Y-shape, into a dorsal longitudinal vein, extending inferiorly, to drain into the venous variceal pouch, previously partially embolized. An attempt was made to access 1 of the 2 smaller fistulas using the smallest braided and flow-guided microcatheters available. Because of the small size of the segmental arteries supplying the ASA and the fistula, access to the ASA was not possible.
During the subsequent 3 months, she again began to experience increasing pain, primarily in the perineal region. The previous radicular right lower extremity pain had not recurred.
The case was discussed at the multidisciplinary neurovascular conference. Given the relative youth of the patient, and the progressive nature of the symptoms, treatment was recommended. Given the multiplicity of pial fistulas (3 total), the categorization of the 2 additional fistulas as type IVa, and the location of the 2 remaining fistulas on the dorsal surface of the cord, surgical treatment was recommended. The patient underwent open surgical treatment 8 months after her first admission to our center. The fistula was approached through a T11–L1 laminectomy. Intradural exposure of the fistula revealed the coil mass packed into the inferior aspect of the varix with persistent arterialized flow as expected from the presurgical angiogram. Inspection of the varix revealed 3 arterialized draining veins with connections to the superior aspect of the fistula as well as several enlarged arteries coursing inferiorly toward the conus. Numerous nerve roots and the varix mass made identification of the arterial venous varix connections difficult. Temporary vascular clips were placed across the arteries and the varix was checked with a flow Doppler for persistent flow. The presence of flow despite the arterial occlusions suggested that the fistula had numerous smaller connections and would require skeletonization and removal of the varix to eliminate the shunt surgery. A number of small AVF connections were encountered that were coagulated and divided as the entire varix was mobilized out of the conus. With the removal of the varix and elimination of the arterialized flow, the normal conus veins darkened, serving as a direct indicator that the fistula had been successfully eliminated. Further inspection in the region of the conus revealed what was felt to be a true small AVM that was resected as well. Postprocedure angiography confirmed that the fistula was completely obliterated. The patient had some postoperative bowel and bladder dysfunction that improved significantly on subsequent follow-up.
Case 2
The patient was a 29-year-old woman with known KTW syndrome, with a history of multiple documented venous malformations involving the extremities, chest wall, spleen, and pulmonary parenchyma with pulmonary vein varicosities. The venous malformation in the left posterior chest wall had presented with left chest wall pain and discomfort and had been successfully treated by embolization 4 years before. Approximately 2 months before her office visit, she had experienced a sudden onset of neck pain that occurred after her right upper extremity was pulled hard while she was walking her dog. Approximately 2 weeks later, she began to experience significant right upper extremity weakness and numbness, primarily involving the hand and forearm, with relatively sparing of the shoulder girdle.
MR imaging of the cervical and thoracic spine with and without contrast performed at an outside institution demonstrated a prominent epidural lesion that extended from the level of C5 down to T2. The signal intensity characteristics were compatible with an epidural hematoma centered in the right posterolateral aspect of the spinal canal, causing mass effect on the thecal sac and cord and the right C8 and T1 nerve roots (Fig 2). The patient was placed on oral corticosteroids because of the obvious mass effect on the cord, and surgical decompression was advised. An early appointment was made for her to see a vascular neurosurgeon (C.C.G.).
Case 2. Sagittal postcontrast fat-saturated T1-weighted images demonstrating partly enhanced extradural lesion in the dorsal epidural space extending from C5 to T2 with mass effect on the thecal sac (arrow, A). Axial T2-and T1-weighted MR imaging demonstrates a right dorsolateral epidural lesion of mixed signal intensity, consistent with a hematoma and probable vascular channels (arrows, B and C). Arch injection demonstrating an AVM (arrow) involving the cervical spine with venous drainage into the epidural venous (curved arrows, D) and subsequently the paraspinous veins (arrowheads, D). Right vertebral artery injection demonstrating a fistulous arteriovenous connection to a dilated multilobulated epidural vein (arrow, E) secondarily draining into extraspinal veins via a stenotic connection (curved arrow, E). Selective right costocervical (deep cervical branch) injection demonstrating supply to the AVM (F). Selective left costocervical (deep cervical branch) injection demonstrating supply to the AVM (G).
Although all of her malformations to date had been venous, a decision was made to better identify the type of vascular malformation before treatment. At first, a time-resolved MRA was performed (Fig 2) that demonstrated definite arteriovenous shunt surgery in the cervical epidural space, thus excluding a pure venous malformation and suggesting an AVM. A conventional arteriogram was performed. An aortic arch injection demonstrated an AVM within the spinal canal extending from the top of C6 to the bottom of T1, with the nidus located in the right dorsolateral aspect of the spinal canal. The AVM drained via the paraspinous veins superiorly and inferiorly. Arterial supply to the AVM arose from segmental branches of the right vertebral artery, as well as the right and left costocervical trunks. The venous drainage included a varix in the right posterolateral epidural space, with subsequent extraspinal drainage extending superiorly, as well as a smaller separate vein draining into the ventral epidural venous plexus and then the right external jugular vein. There was a focal stenosis at the connection of the epidural varix with the extraspinal veins. The venous varix caused erosion and widening of the right C6/C7 and C7/T1 intervertebral foramina. After careful consideration, endovascular embolization of the lesion followed by surgical resection was recommended to the patient.
Embolization of the AVM was performed in 2 sessions. In the first session, the right-sided supply from the costocervical trunk was embolized using a combination of polyvinyl alcohol (PVA) particles and n-BCA. In the second session, the left costocervical trunk supply was embolized using a combination of PVA particles and n-BCA. After the final session, the lesion was nearly completely devascularized. No complications were encountered.
The patient underwent surgical removal of the AVM with laminectomies at C5–T2 the day after the last embolization. The objective of surgery was to decompress the spinal cord at the level of the malformation with resection of as much of the vascular tissue as feasible. A bilateral cervical laminectomy was performed from C5–T2. A significant amount of bleeding was encountered during the exposure of the lamina from numerous emissary veins coursing through the T1 lamina. Inspection of the epidural space upon completion of the laminectomies revealed a thick plaque of arterialized dilated vascular channels extending from C6 to T2 where the tissue tapered out. The tissue was divided carefully in the midline using bipolar electrocautery with controllable bleeding. The tissue peeled easily laterally to the edges of the laminectomies, where it was amputated and sent en bloc for pathologic examination. The surface of the dura also contained engorged venous channels that were coagulated. There was no evidence of the malformation extending into the intradural compartment. One small connection from the dural venous channels to the epidural vascular malformation was coagulated and divided. Hemostasis was achieved, and the incision was closed. A postoperative arteriography was performed revealing complete elimination of the AVM.
Discussion
KTW syndrome consists of a classic triad of venous varices, cutaneous vascular nevi, and limb hypertrophy and may include AVMs. The KTW syndrome is generally thought to occur sporadically, following a somatic mutation model. However, in some cases, clinical manifestations of the syndrome have been found in family members, suggesting an autosomal dominant inheritance.6 Ceballos-Quintal et al7 reported KTW syndrome in a family where the family tree supported an autosomal dominant inheritance. There are also some reports of chromosomal abnormalities in some cases of KTW syndrome.8–10 These observations all suggest a genetic contribution to the occurrence of KTW syndrome with multiple possible genes involved. Timur et al10, 11 cloned a susceptibility gene (named AGGF1) for KTW syndrome. AGGF1 encodes a potent angiogenic factor. KTW syndrome-associated mutations enhance the activity of this gene, suggesting increased angiogenesis as one molecular mechanism for the pathogenesis of this syndrome.11Some authors have grouped this syndrome with neurocutaneous hereditary diseases such as Von Hippel-Lindau, neurofibromatosis, Sturge-Weber syndrome, and tuberous sclerosis.1, 3 Furthermore, several case reports showed no clear distinction between the KTW syndrome and related entities such as Sturge-Weber syndrome or Proteus syndrome. Vascular malformations are a common feature in all these syndromes, malformations that might be due to gene mutations that cause dysregulation in the signaling involved in vascular morphogenesis, growth, and development.10, 12–14
The presentations of this syndrome are variable. The cutaneous vascular nevi can be in the form of port-wine stains, hemangiomas, or lymphangiomas. In some subsets of patients, on the other hand, only 2 of the 3 classic findings are present.
Our first patient had partial hypertrophy of a lower limb with associated varices; however, cutaneous capillary malformations were conspicuously absent. Our second patient had significant hypertrophy of the right lower extremity with associated bony abnormalities. The venous abnormalities were not limited to a single extremity; rather, they involved the trunk and all 4 extremities.
The association of KTW syndrome and spinal cord vascular lesions has been reported in the medical literature. Djindjian et al5 described 5 cases of KTW syndrome associated with intramedullary spinal cord AVMs discovered at a variable time after the diagnosis of KTW syndrome. Benhaiem-Sigaux et al15 reported a case of this syndrome associated with a retromedullary spinal AVM. Alexander et al3 reported a case of extradural thoracic AVM in a known case of KTW syndrome. To the best of our knowledge, 22 reported cases of the KTW syndrome are associated with spinal vascular malformations (Table).3, 5, 15–29
Summary of reported cases of Klippel-Trenaunay-Weber Syndrome associated with spinal arteriovenous malformations
The selection of treatment modalities depends primarily on the type of spinal vascular malformation encountered, and a discussion of treatment for the various types of malformations is beyond the scope of this study. Treatment modalities include microsurgery, endovascular embolization, and, more recently, stereotactic radiosurgery. Concerning the treatment of perimedullary fistulas (ventral epidural or type IV), endovascular treatment has the primary role for treatments of subtypes B and C, as a result of the enlargement of the ASA, which allows for microcatheter access. Patients presenting with subtype A are usually considered better microsurgical candidates due to the small size of the ASA, which often precludes microcatheter navigation. Recently however, Oran et al31 reported successful endovascular treatment of 4 patients with Anson-Spetzler type IV, subtype A perimedullary fistulas. With regard to the treatment of epidural AVMs, microsurgical removal is necessary in a case such as ours in which the patient presented with hemorrhage and mass effect on the spinal cord and exiting nerve roots. Preoperative embolization was thought to be necessary to make the operation safer and faster.
The recognition and proper categorization of spinal AVMs are crucial for planning the right treatment options. Because of the small numbers of published series, there is no consensus about the classification and optimal treatment options for spinal AVMs in KTW syndrome. Spetzler et al32 introduced a modified classification for spinal AVMs in 2002. This more recent classification divides spinal AVMs into extradural-intradural and intradural types. The former type—previously known as “juvenile” or Anson-Spetzler type III—is uncommon, usually does not respect any tissue boundary, and usually needs a multidisciplinary approach consisting of embolization and surgical resection. The intradural AVMs are further classified into intramedullary, intramedullary-extramedullary, and conus medullaris subtypes. The intramedullary subtype (previously known as glomus AVMs or Anson-Spetzler type II) can be supplied by multiple branches of the anterior and posterior spinal arteries and are characterized by high pressure, low resistance, and high blood flow. The conus medullaris AVMs are always located in the conus medullaris and cauda equina and, unlike other spinal arteriovenous lesions, frequently produce radiculopathy and myelopathy at the same time. Spetzler et al32 also modified the classification for the spinal AVF; these are classified as extradural (formerly known as epidural), dorsal intradural (formerly known as Anson-Spetzler type I), and ventral intradural (previously known as Anson-Spetzler type IV). A detailed discussion on the types of fistulas is beyond the scope of this article; however, dorsal intradural fistulas are the most common type of spinal AVF and usually occur in thoracic and lumbar region. They are further subdivided to type A (single feeding artery) and type B (multiple feeding arteries). Ventral intradural fistulas originate directly from the ASA and have a direct fistula to an enlarged venous network. These lesions are further divided to subtypes A, B, and C depending on the size of the shunts.31
Nakstad and colleagues24 presented 4 spinal AVFs discovered at the same time in a 13-year-old patient with known KTW syndrome and embolized with platinum fiber coils. The first patient reported in our study thus represents only the second reported case of multiple spinal AVFs in association with KTW syndrome.
We report 2 rare cases: a patient with 3 concurrent spinal perimedullary fistulas and another with a spinal epidural AVM. These cases and our review of the medical literature emphasize 2 important points regarding patients with KTW presenting with spinal vascular malformations. First, although the predominant vascular abnormalities in these patients consisted of venous and or lymphatic abnormalities, when the spine is involved, there is a high incidence of arteriovenous shunts, and appropriate investigation with MRA, CTA, or conventional angiography is indicated. Second, it is extremely important to evaluate these patients for the presence of more than one spinal vascular malformation.
References
- Received March 2, 2006.
- Accepted after revision April 6, 2006.
- Copyright © American Society of Neuroradiology