Abstract
BACKGROUND AND PURPOSE: Pediatric upper airway carcinoma is uncommon, symptoms are nonspecific, and diagnosis is often delayed. In this study, we describe the imaging, cytogenetics, and clinical courses of 4 patients with pediatric upper airway carcinoma.
MATERIALS AND METHODS: Four patients with upper airway carcinoma were identified during a 2.5-year period. CT (n = 4) and MR imaging (n = 3) studies, tumor histopathologic features and cytogenetics, patient treatment, and clinical course were reviewed.
RESULTS: Patients were aged 12 to 15 years. One tumor involved the larynx with poorly defined margins and heterogeneous enhancement; 1 heterogeneously enhancing tumor involved the epiglottis with necrotic cervical lymphadenopathy. There were 2 enhancing sinonasal tumors with bony destruction in 1 tumor. Tumors had a relatively short relaxation time on FSEIR MR imaging. Histopathologic examination revealed poorly differentiated squamous cell carcinoma (n = 3) and well-differentiated squamous cell carcinoma (n = 1). Cytogenetic analysis revealed chromosomal abnormalities in 3 tumors: 2 showed a chromosomal translocation t(15;19), and 1 showed a chromosomal translocation t(1;5) and loss of a portion of chromosome 22q. Results of in situ hybridization for EBV were negative (n = 3). Treatment included tumor resection (n = 2), chemotherapy (n = 4), and radiation therapy (n = 3). Patients with t(15;19) died months after diagnosis. Two patients were alive at 8-year follow-up.
CONCLUSIONS: Childhood carcinoma of the upper airway is uncommon but should be considered in the diagnosis of upper airway tumors that display aggressive imaging characteristics. Carcinoma with t(15;19) is rare but has been reported, usually in young patients with midline carcinoma of the neck or mediastinum, with a rapidly fatal course.
Abbreviations
- EBV
- Epstein-Barr virus
- EBER
- Epstein-Barr virus encoded RNA
- FSEIR
- fast spin-echo inversion recovery
- FISH
- fluorescent insitu hybridization
- f/u
- follow-up
- Gy
- Gray
- LN
- lymph nodes
- mos
- months
- msEF
- effective milliseconds
- NPC
- nasopharyngeal carcinoma
- URT
- upper respiratory tract
- VCR
- vincristine
- wk
- weeks
- y
- year.
Carcinoma of the upper aerodigestive tract is rare in children, and symptoms are generally nonspecific, often resulting in a delay in diagnosis. The incidence and prevalence of carcinoma of the upper aerodigestive tract, excluding the nasopharynx, are not known. Lymphoma (52%–59% of cases) and rhabdomyosarcoma (13%–22% of cases) account for most cases of malignant tumors of the head and neck in children.1,2 Thyroid, nasopharyngeal, and salivary gland carcinomas are the most frequently encountered pediatric head and neck carcinomas. In children, the most frequently encountered destructive primary tumors in the sinonasal region include juvenile nasopharyngeal angiofibroma (JNA) and sarcoma (including rhabdomyosarcoma). JNA is generally readily diagnosed on the basis of characteristic history and imaging findings. Tumors of the epiglottis and glottis are uncommon in children. Benign tumors in this region include laryngeal neurofibroma, hemangioma, and laryngeal papilloma. Malignant tumors of the epiglottis and larynx are unusual and include various types of carcinoma, sarcoma, and primitive neuroectodermal tumor.3 To date, there are only isolated case reports and small series of sinonasal and laryngeal carcinoma in children, to our knowledge.4–8 Our purpose was to describe the imaging characteristics of a small series of children with carcinoma of the upper aerodigestive tract and to correlate these findings with clinicopathologic and cytogenetic features. In all patients, imaging features were helpful in excluding some of the more commonly encountered lesions in these locations.
Materials and Methods
During a 2.5-year period (1999–2001), a total of 4 patients at our institution presented with masses involving the upper aerodigestive tract and were diagnosed with carcinoma, located in the sinonasal region in 2, the larynx in 1, and the epiglottis in 1. Institutional review board approval was obtained for this study of those patients. The medical records and imaging studies were reviewed for the 4 patients (3 girls and 1 boy), who ranged in age from 12 to 15 years at the time of presentation. A lateral radiograph of the neck was obtained in 1 patient, a CT scan in all patients, and MR imaging in 3 patients.
All study patients underwent a CT scan of the head and neck from the skull base to the thoracic inlet with an Advantage CT scanner (GE Healthcare, Milwaukee, Wisconsin). Patients were scanned with 3- to 5-mm sequential axial images (n = 2) or 3-mm coronal images (n = 2). CT scan parameters were 120 kV, 200 mAs, a 20- to 25-cm FOV, and a 512 × 512 matrix. Contrast material consisted of an intravenous injection of 2 mL/kg of Ioversol 68% (w/v) (Optiray 320; Mallinckrodt Medical, St. Louis, Missouri).
Three patients underwent MR imaging of the head and neck on a 1.5T system (GE Healthcare) with use of a volume neck coil. The imaging protocol consisted of sagittal and axial conventional spin-echo T1-weighted images (TR, 450–700 ms; TE, 14 msEf; NEX, 2; section thickness, 3–5 mm; intersection gap, 1 mm; FOV, 24 cm; matrix, 512 × 192); axial and coronal FSEIR images (TR, 4000 ms; TE, 32 msEf; inversion time, 150 ms; NEX, 2; section thickness, 3–5-mm; intersection gap, 1 mm; FOV, 22–24 cm; matrix, 512 × 192); and gadolinium-enhanced (0.1 mmol/kg of gadopentetate dimeglumine [Magnevist; Berlex Laboratories, Wayne, New Jersey]), fat-suppressed axial, coronal, and sagittal T1-weighted images (parameters as above).
The images were reviewed by a pediatric neuroradiologist for location and extent of tumor, attenuation and signal intensity characteristics, pattern of enhancement, bony erosion, and regional metastatic disease. All tumor measurements are approximate maximal anteroposterior; transverse; and, if available, superoinferior dimensions.
Results
Clinical history, radiographic and pathologic findings, tumor cytogenetics, treatment, and clinical course are summarized in the Table.
Patient results
Patient 1.
A 13-year-old adolescent girl presented with a 6-week history of sore throat, odynophagia, and subsequent voice change, with an enlarging neck mass that arose 12 days before admission. There was no medical or family history of note. Physical examination revealed a large, pale, irregular fungating mass located posterior to the base of the tongue. There was also a tender, mobile 3 × 6-cm mass on the left side of her neck.
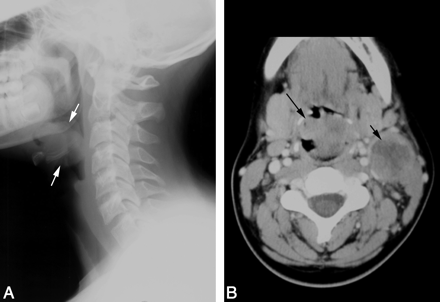
A lateral radiograph of the neck revealed a rounded, irregular mass in the region of the epiglottis (Fig 1A). Contrast-enhanced CT scan from the same institution revealed that the irregular, heterogeneously enhancing, 37-mm × 30-mm midline mass was arising from the epiglottis and aryepiglottic folds, extending into the hypopharynx, with significant narrowing of the airway. The tumor abutted the inner cortex of the hyoid bone. There was a conglomerate, heterogeneously enhancing necrotic mass of enlarged level II lymph nodes on the left, measuring 28 × 32 mm (Fig 1B). CT scan of the chest, abdomen, and pelvis revealed no metastatic disease.
Patient 1. Carcinoma of the epiglottis; t(15;19). A, Lateral film of the neck reveals an irregular epiglottic mass (arrows). B, Contrast-enhanced neck CT scan demonstrates the irregular, midline epiglottic mass (long arrow). There is necrotic left level II adenopathy (short arrow).
The patient underwent tracheostomy, direct laryngoscopy, and biopsy. The tumor arose from the left side of the epiglottis, extending to the left aryepiglottic fold. Histopathologic examination of the tumor revealed undifferentiated carcinoma. Cytogenetic analysis revealed the following karyotype: 47,XX, +8, t(15;19)(q13;p13.1). Results of in situ hybridization for EBER in tumor cells were negative. The pathologic features are described in further detail elsewhere.8
The patient was treated with chemotherapy and radiation therapy (Table). This was followed by modified radical dissection on the left side of the neck and additional chemotherapy. However, there was relentless local tumor progression and tumor dissemination to the chest wall, metastatic adenopathy, and parenchymal metastases to the lung. The patient died as a result of this tumor approximately 10 months after diagnosis.
Patient 2.
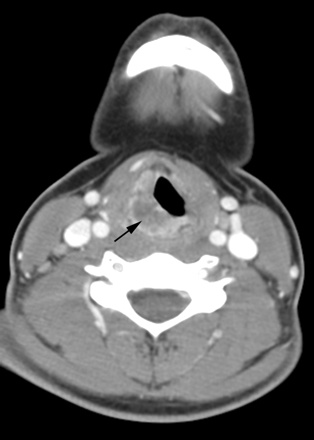
A 14-year-old adolescent girl presented with a 4-month history of hoarseness and sore throat and a 1-month history of odynophagia and dysphagia. Clinical examination revealed a mass at the level of the glottis on the right. Contrast-enhanced CT scan (Fig 2) and MR imaging revealed a 28-mm × 13-mm × 31-mm irregular and heterogeneously enhancing, submucosal mass involving the right side of the epiglottis and aryepiglottic fold, extending inferiorly to involve the right side of the larynx. The mass exhibited a relatively short T2 relaxation time but was hyperintense relative to muscle, with the pulse parameters used on the FSEIR images. There were mildly asymmetric, enhancing ipsilateral level IV and V lymph nodes that did not meet size criteria for pathologic enlargement. On fiberoptic examination, the mass appeared to be bulging into the right pyriform sinus, causing fixation of the right vocal cord, with minimal subglottic extension. Results of biopsy revealed a well-differentiated invasive squamous carcinoma. The tumor was classified as T3N0M0. Fresh tissue was not submitted for karyotypic analysis; results of paraffin-embedded tissue was negative by FISH for t(15;19). In situ hybridization for EBER was not performed.
Patient 2. Carcinoma of the larynx. Contrast-enhanced CT scan of the neck demonstrates an irregular and heterogeneously enhancing, off-midline submucosal mass (arrow) involving the right side of the larynx.
The patient required a tracheostomy and gastrostomy tube during the treatment period and underwent radiation therapy for local control to bilateral neck nodes. This was followed by chemotherapy (Table). Five years after diagnosis, the patient remained tracheostomy dependent and underwent a partial right hemilaryngectomy for treatment-related scarring. Eight years after diagnosis, the patient was disease free with a clinical course complicated by scarring of the right hemilarynx and supraglottis, with laryngeal and subglottic stenosis.
Patient 3.
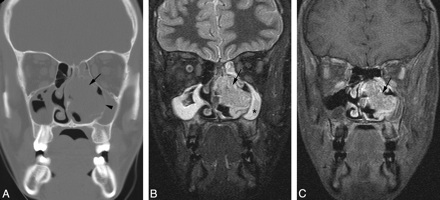
A 15-year-old adolescent boy presented with nasal obstruction and was found to have a nasal mass by an outside institution. Contrast-enhanced CT scan demonstrated a 40-mm × 29-mm × 29-mm lobulated, moderately enhancing mass arising from the left side of the nasal cavity. The mass eroded the middle turbinate and caused marked thinning and lateral deviation of the medial wall of the left maxillary antrum, and deviation of the nasal septum (Fig 3A). There was elevation of the inferomedial wall of the left orbit but no evidence of extension of tumor into the orbit. The tumor exhibited a relatively short T2 relaxation time but was hyperintense relative to muscle, with the pulse parameters used on the FSEIR images. It was hypointense compared with the trapped secretions in the ipsilateral maxillary antrum (Fig 3B). The tumor enhanced homogeneously (Fig 3C). The ipsilateral maxillary antrum and ethmoid air cells appeared obstructed. Biopsy results revealed undifferentiated carcinoma. Results of in situ hybridization for EBER were negative. Cytogenetic analysis revealed the following karyotype: 46,XY,+der(1)t(1;5)(p12;q11.1),−5,del(22)(q11q13).
Patient 3. Sinonasal carcinoma. A, Coronal CT scan of the sinuses reveals a tumor (arrow) arising from the left nasal cavity. The mass causes marked thinning and lateral deviation of the medial wall of the left maxillary antrum (arrowhead) and rightward deviation of the nasal septum. B, Coronal FSEIR MR imaging demonstrates that the tumor (arrow) is hypointense compared with the trapped secretions in the left maxillary antrum (star). C, Gadolinium-enhanced, fat-suppressed coronal T1-weighted MR image shows that the tumor (arrow) enhances homogeneously.
The patient received chemotherapy (Table) and approximately 8 months after diagnosis underwent excision of the left sinonasal mass. Histopathologic examination revealed poorly differentiated squamous carcinoma. The postoperative course was uncomplicated. Eight years after diagnosis, the patient was free of disease.
Patient 4.
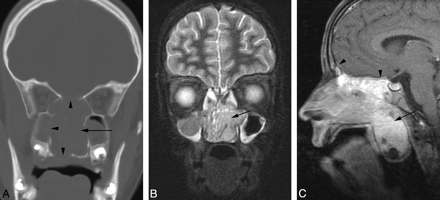
A 12-year-old girl presented with 3 weeks of upper respiratory tract symptoms, having been treated with 2 courses of antibiotics without improvement. Contrast-enhanced MR imaging revealed an 83-mm × 41-mm × 60-mm mass located in the midline within the nasal cavity (Fig 4). The mass obstructed the nasopharynx and protruded into the upper oral cavity. Superiorly and laterally, it extended into the contiguous paranasal sinuses. There was erosion of the planum sphenoidale and adjacent dural enhancement. The tumor had a relatively short T2 relaxation time but was hyperintense relative to muscle, with the pulse parameters used on the FSEIR images, and enhanced avidly apart from a few small cystic or necrotic components. CT scan obtained 11 days after the MR imaging examination demonstrated a moderately enhancing mass located in the midline within the nasal cavity, with lytic destruction of the contiguous paranasal sinuses and right orbit, extension into the right orbital apex, and erosion of the palate and pterygoid plates (Fig 4). There was erosion of the central skull base with tumor progression and intracranial spread of tumor into the epidural space overlying the planum sphenoidale. The maxillary antra and ethmoid air cells appeared opacified because of obstructed secretions. Biopsy results revealed poorly differentiated squamous carcinoma. Results of in situ hybridization for EBER were negative. FISH showed t(15;19). The pathologic features are described in further detail elsewhere.8
Patient 4. Sinonasal carcinoma t(15;19). A, CT scan demonstrates a midline sinonasal tumor (arrow) with lytic bony destruction of the paranasal sinuses and hard palate (arrowheads). B, Coronal FSEIR MR demonstrates the tumor (arrow), which had a relatively short T2 relaxation time but was hyperintense relative to muscle. C, Sagittal gadolinium-enhanced, fat-suppressed T1-weighted MR image demonstrates enhancing tumor (arrow) extending into the nasopharynx. There is dural enhancement superiorly (arrowheads).
The patient received radiation therapy and chemotherapy (Table). However, there was rapid development of disseminated spinal and pelvic metastatic disease and subsequent death approximately 3 months after diagnosis.
Discussion
Carcinoma of the upper aerodigestive tract is uncommon in children.2 Although this small series of patients presented within a 2.5-year period, we have seen a total of only 6 such tumors during a 15-year period. Pediatric carcinoma most often presents in the latter half of childhood, as illustrated by our patients who ranged from ages 12 to 15 years. Symptoms are often nonspecific and can mimic an upper respiratory tract infection. As a result, diagnosis is frequently delayed, ranging from 3 weeks to 4 months in our patients. In this small series, the clinical finding of a mass involving the upper airway with imaging findings of a destructive or infiltrative process are suggestive of a high-grade neoplasm. In all patients, imaging features were helpful in excluding some of the more commonly encountered lesions in these locations but did not differentiate between other high-grade tumors such as lymphoma or sarcoma. Aggressive features on imaging were well illustrated by patients 1 and 4, who had poorly differentiated midline carcinomas with t(15;19) and died after treatment, approximately 3 to 10 months after diagnosis. The sinonasal tumor in patient 4 demonstrated extensive lytic destruction of the bone of the paranasal sinuses and central skull base. The signal intensity of the tumor on FSEIR MR images suggested a cellular neoplasm such as carcinoma, sarcoma, or lymphoma in 3 of 3 patients. Imaging in patient 1 revealed an epiglottic mass with obvious asymmetric necrotic adenopathy for which the imaging features, despite the young age of the patient, were suggestive of carcinoma.
Patients 2 and 3 lacked the t(15;19) chromosomal translocation. Although the imaging appearance of the tumors in these patients appeared less aggressive, the number of patients is far too low to provide any proof of correlation. These patients have remained disease-free after treatment for approximately 8 years since diagnosis. Patient 2 demonstrated unusual features of asymmetric mucosal and submucosal thickening of the epiglottis and larynx, with low attenuation that was interpreted initially as edema. The radiographic differential diagnosis included inflammatory or granulomatous processes as well as neoplasms such as lymphoma. Patient 3 demonstrated bony remodeling of contiguous sinuses on CT; however, MR imaging again showed relative tumor hypointensity suggestive of the highly cellular nature of the tumor.
The most commonly encountered carcinoma of the upper aerodigestive tract in children during the second decade of life is NPC.1,2 NPC occurs in a similar age group to these patients and is strongly associated with previous EBV infection, as evidenced by the detection of latent EBV in tumor cells with use of EBER in situ hybridization.9,10 The carcinomas in this series were not associated with EBV.
In 2 of our cases, an unusual t(15;19) chromosomal aberration was detected. These patients had rapid clinical deterioration and died of disseminated disease despite treatment. This chromosomal aberration has been previously reported by our group.8,11 Patients with this chromosomal translocation are usually young, develop midline carcinoma of the head and neck or upper mediastinum, and have a rapidly fatal course despite intense treatment. More recently, the molecular events associated with the t(15;19) translocation have been elucidated. The translocation results in a novel fusion oncogene involving the bromodomain gene BRD4 and the nuclear protein in testis gene.12 In contrast to the patients with t(15;19), the patient with t(1;5) exhibited extended disease-free survival duration. This suggests that the tumorigenic mechanisms in this translocation are less aggressive.
Conclusions
This small series illustrates the imaging features of carcinoma involving the upper airway in children. This uncommon entity should be considered in the differential diagnosis of upper airway tumors that display aggressive imaging characteristics. Carcinoma with t(15;19) is rare but has been reported, usually in young patients with midline carcinoma of the neck or mediastinum, with a rapidly fatal course, and this highlights the emerging importance of chromosomal studies in pediatric carcinomas.
Footnotes
Previously presented at: Annual Meeting of the American Society for Neuroradiology, Vancouver, Canada, May 17, 2002.
References
- Received March 13, 2009.
- Accepted after revision July 1, 2009.
- Copyright © American Society of Neuroradiology