Abstract
BACKGROUND AND PURPOSE: The vascular supply of extra-axial brain tumors provided by the external carotid artery has not been studied with RPI. The purpose of this work was to determine whether RPI assessment is feasible and provides information on the vascular supply of hypervascular extra-axial brain tumors.
MATERIALS AND METHODS: Conventional ASL and RPI studies were performed at 3T in 8 consecutive patients with meningioma. On the basis of MRA results, we performed RPI by placing a selective labeling slab over the external carotid artery. Five patients underwent DSA before surgery. Two neuroradiologists independently evaluated the overall image quality, the degree of tumor perfusion, and the extent of the tumor vascular territory on conventional ASL and RPI.
RESULTS: In overall quality of conventional ASL and RPI, no images interfered with interpretation. In comparisons of the vascular tumor territory identified by the conventional ASL and RPI techniques, the territories coincided in 3 cases, were partially different in 4, and completely different in 1. The interobserver agreement was very good (κ = 0.82). In 5 patients who underwent DSA, the 4 patients in whom the dominant supply was the external carotid artery were scored as coincided or partially different. The 1 patient in whom the vascular supply was from the internal carotid artery was scored as completely different.
CONCLUSIONS: RPI with selective labeling of the external carotid artery is feasible and may provide information about the vascular supply of hypervascular extra-axial brain tumors.
Abbreviations
- ACA
- anterior cerebral artery
- APA
- ascending pharyngeal artery
- ASL
- arterial spin-labeling
- CD
- completely different
- DSA
- digital subtraction angiography
- FLAIR
- fluid-attenuated inversion recovery
- ICA
- internal carotid artery
- Lt
- left
- MMA
- middle meningeal artery
- MRA
- MR angiography
- NSA
- number of signal-intensity acquisitions
- PCA
- posterior cerebral artery
- PD
- partially different
- PULSAR
- pulsed star labeling of arterial regions
- Q2TIPS
- second version of quantitative imaging of perfusion using a single subtraction with thin-section TI1 periodic saturation
- QUASAR
- quantitative STAR labeling of arterial regions
- RPI
- regional perfusion imaging
- Rt
- right
- TFE
- turbo field echo
ASL MR imaging is a noninvasive technique to depict brain tissue perfusion without using exogenous contrast material. Among ASL-MR imaging methods, RPI provides selective information on the vascular territory of individual brain-feeding arteries.1–6 To our knowledge, the vascular supply of extra-axial brain tumors provided by the external carotid artery has not been studied with RPI. The hypotheses in our study were the following: 1) RPI is feasible for depicting tumor perfusion of hypervascular extra-axial brain tumors, and 2) RPI findings correlate with the vascular supplies seen during selective catheter DSA. The purpose of our study was to verify these hypotheses by using the MR imaging data in 8 patients and DSA data in 5 patients who underwent DSA.
Materials and Methods
Patients
Prior written informed consent for MR imaging studies was obtained from all patients. Our study was approved by the institutional review board of our hospital. Conventional MR imaging, MRA, ASL, and RPI studies were performed in 8 consecutive patients (5 women and 3 men) with hypervascular extra-axial brain tumors. Their ages ranged from 42 to 73 years (mean, 59 years). The characteristics of the patients and tumors are shown in Table 1. After the MR imaging studies, all 8 patients underwent surgery; 5 underwent DSA before surgery. Preoperative embolization was performed in 2 patients (cases 3 and 7). The tumors included 7 meningiomas and 1 malignant meningioma.
Summary of patient characteristics and image findings in 8 patients with meningioma
Conventional MR Imaging and MRA
All MR imaging studies were performed on a 3T scanner (Achieva 3.0T; Philips Medical Systems, Best, the Netherlands) by using 8-channel head coils. The imaging sequences included 3-plane scout localizers, axial spin-echo T1-weighted (TR/TE/NSA, 450 ms/10 ms/1; matrix, 320 × 320), turbo spin-echo T2-weighted (TR/TE/NSA, 4060 ms/80 ms/1; turbo factor, 9; matrix, 512 × 512), FLAIR (TR/TE/NSA/TI, 9000 ms/120 ms/1/2500 ms; turbo factor 15; matrix, 352 × 352), and postcontrast T1-weighted images. The FOV was 23 cm on all conventional MR images.
Before the contrast-enhanced MR imaging studies, we performed 3D time-of-flight MRA to evaluate the intracranial arteries with the following parameters: TR/TE/NSA, 20 ms/3.5 ms/1; flip angle, 20°; 0.5-mm-thick sections; FOV, 20 cm; matrix, 512 × 208; effective voxel size, 0.39 × 0.96 × 0.5 mm; and acquisition time, 4 minutes 48 seconds. Cephalad saturation pulses were applied to eliminate venous blood signals. For localization of RPI scans, 3D phase-contrast MRA was also performed to demonstrate the external carotid artery with the following parameters: TR/TE/NSA, 14 ms/3.5 ms/1; flip angle, 12°; 1.5-mm-thick sections; FOV, 20 cm; matrix, 192 × 192; and acquisition time, 4 minutes 46 seconds.
Conventional ASL and RPI MR Imaging Studies
Before contrast-enhanced MR imaging studies, conventional ASL and RPI scans were acquired by using the same MR imaging scanner. We used the QUASAR sequence7; it combines a PULSAR labeling technique1 with a Look-Locker readout for sampling at multiple TI points8 and a Q2TIPS-like bolus saturation scheme for clear definition of the arterial blood bolus.9,10 Each section acquisition was preceded by a bolus saturation of a slab, applied inferior to the volume of interest.
We referred to T2-weighted and FLAIR images to select 5 axial images through the tumor for conventional ASL and RPI studies. The imaging parameters for conventional ASL imaging were the following: TR/TE, 3000 ms/ 24 ms; flip angle, 30°; sensitivity encoding factor, 2.5; FOV, 23 × 23 cm; matrix, 64 × 64 (3.59 × 3.59 mm in-plane resolution); and section thickness/gap, 6 mm/2 mm. The labeling delay time (TI) was 40 ms. In each series of the ASL sequence, 7 different phase images were acquired every 250 ms (Δ TI = 250 ms). A total of 40 images, labeled-subtracted from nonlabeled images, were obtained. The labeling slab thickness was 150 mm; it was positioned at the level of the upper cervical region. The total acquisition time was 4 minutes 12 seconds
On the basis of the 3D phase-contrast or MRA results, we performed RPI. We used a regional perfusion imaging sequence.2 We placed a selective labeling slab over the external carotid artery by using a single-shot echo-planar imaging sequence (TR/TE, 3000 ms/22 ms; spin-echo acquisition and sensitivity encoding factor, 2.5; matrix, 64 × 64; 40 dynamics; 7 sections; section thickness, 6 mm; scanning time, 4 minutes 23 seconds) (Fig 1). Selective labeling was obtained by using the sharp labeling profiles of the transfer insensitive labeling technique pulses11 and by interactively planning the spatially selective inversion slab to invert the targeted artery only. The labeling slab thickness was 40 mm. The TI and Δ TI of RPI were the same as those of the conventional ASL sequence.
On the basis of MR angiography results, we acquired regional perfusion images by placing a selective labeling slab over the external carotid artery.
DSA Procedure
Five of the 8 patients underwent DSA; vascular access was acquired by using the transfemoral approach and the Seldinger technique. The vascular anatomy was evaluated by DSA combined with the selective injection of iodinated contrast medium into the external and internal carotid arteries and the vertebral arteries. This procedure was bilateral in all patients, irrespective of the tumor location.
Image Analysis
One rater (T.H., with 20 years of experience in neuroimaging) qualitatively evaluated all DSA images on a PACS workstation. The feeding artery of the tumors was identified on DSA images.
The conventional ASL and RPI maps were calculated on a pixel-by-pixel basis by using built-in software on the MR imaging unit. Two other raters (K.M. and T.O., with 18 and 20 years of experience in neuroradiologic MR imaging, respectively), who were blinded to clinical and DSA results, independently evaluated the conventional ASL and RPI data at a PACS workstation. In the 2 imaging studies, the 2 raters analyzed a total of 40 images generated from labeled and control images. They evaluated the overall image quality, the degree of tumor perfusion, and the extent of the vascular tumor territory. Each observer performed the initial evaluations independently; disagreements regarding final conclusions were resolved by consensus.
At first, the overall image quality of the conventional ASL and RPI studies was recorded by using a 3-point scale: class 3, class 2, and class 1. Class 3 meant that images had sufficient quality for interpretation and no or slight artifacts. Class 2 indicated that images had mild-to-moderate artifacts not interfering with interpretation. Class 1 meant that image quality was inadequate, and there were severe artifacts interfering with interpretation.
From among the 40 conventional ASL images, the observers chose the image with maximum tumor perfusion compared with normal-appearing brain tissue. They qualitatively graded the degree of tumor perfusion by using a 4-point grading system in which grade 3 indicated tumor perfusion higher than that of the normal-appearing cortex; grade 2, tumor perfusion equivalent to that of the normal-appearing cortex; grade 1, tumor perfusion equivalent to that of the normal-appearing white matter; and grade 0, tumor perfusion lower than that of the normal-appearing white matter.
The observers scored the extent of the tumor vascular territory on RPI and conventional ASL images as coincided, partially different, and completely different, where “coincided” indicated that the territory was almost the same; “partially different,” that it was different in some portions; and “completely different,” that it was different in all portions on the 2 types of images. The final interpretation was obtained by consensus. In patients who underwent DSA, the degree of coincidence in the extent of the tumor vascular territory on conventional ASL and RPI was compared with DSA findings.
With respect to the 3 items, the levels of interobserver agreement between rater 1 and rater 2 were determined by calculating the κ coefficient (κ < 0.20 indicated poor agreement; κ = 0.21–0.40, fair agreement; κ = 0.41–0.60, moderate agreement; κ = 0.61–0.80, good agreement; κ = 0.81–0.90, very good agreement; and κ > 0.90, excellent agreement). A statistical package (MedCalc; MediSoftware, Mariakerke, Belgium) was used to perform the calculations.
Results
The overall quality of all conventional ASL images and of 5 of 8 RPI studies was judged to be class 3. In 3 cases (cases 1, 7, and 8), the tumors were located at the posterior fossa or near the skull base. Their image quality on RPI studies was class 2. There were no cases of image quality interfering with interpretation (class 1). The κ values for interobserver variability for conventional ASL and RPI studies showed excellent (κ = 1.0) and moderate agreement (κ = 0.71), respectively.
On conventional ASL studies, both observers rated the perfusion of 7 of 8 tumors higher than that of the normal-appearing cortex (grade 3) (Table 1). In the other case, a patient with posterior fossa meningioma, perfusion was rated as grade 2. The κ values for interobserver variability showed excellent agreement (κ = 1.0).
In comparisons of the tumor vascular territory identified by the 2 techniques, the territories coincided in 3 cases and were partially different in 4 (Table 1). In the other case, the territories were completely different. The κ values for interobserver variability showed very good agreement (κ = 0.82).
Table 2 shows a summary of imaging findings in 5 patients who underwent DSA. In the 2 cases in which the territories were scored as coincided, the feeders derived solely from the external carotid artery. In the 2 cases in which the territories were scored as partially different, the extent of the tumor vascular territory was smaller on RPI than on conventional ASL images (Fig 2). The DSA study in the 2 cases revealed that the feeders derived from both the external carotid and the internal carotid arteries. The vascular supply from the internal carotid artery territory included a parasitic supply to the tumor from the anterior cerebral artery or the posterior cerebral artery and a meningeal artery supply from the internal carotid artery (Fig. 2). In the 1 case in which the territories were scored as completely different, conventional ASL revealed a hypervascular tumor region and RPI showed no apparent tumor vascular territory (Fig 3). DSA in this case revealed that the feeders derived solely from the internal carotid and the ophthalmic arteries.
Summary of imaging findings in 5 patients who underwent DSA
A 58-year-old man with malignant meningioma at the convexity (case 4). A and B, Anteroposterior (A) and lateral (B) projections of the right external carotid angiogram show a hypervascular region (arrows) fed by the right middle meningeal artery. C, Lateral projection of the right internal carotid angiogram shows a parasitic supply from the anterior cerebral artery branches (arrows). The tumor is also fed by the falx artery (arrowhead) from the ophthalmic artery. D, T2-weighted image demonstrates a large mass lesion at the right frontal convexity. E, Conventional ASL image shows a vascular territory with higher perfusion than that in the normal-appearing cortex (arrows). The degree of tumor perfusion is classified as grade 3. F, RPI acquired at the same level as E. The extent of the vascular tumor territory is slightly smaller on the RPI than on the conventional ASL image (arrows). The extent of tumor perfusion on the 2 techniques is classified as partially different.
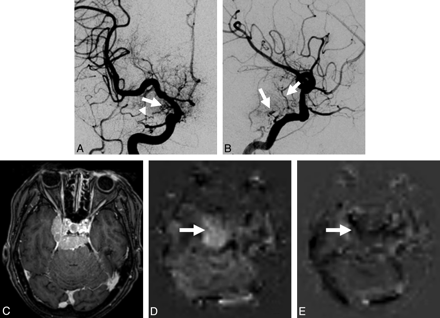
A 71-year-old woman with a meningioma at the right cavernous sinus (case 8). A, Anteroposterior projection of the right internal carotid angiogram shows dilated feeding arteries (arrow and arrowhead) from the internal carotid and ophthalmic arteries, respectively. The external carotid artery branches are also seen due to reflux of contrast medium. B, Lateral projection of the left internal carotid angiogram clearly shows dilated feeding arteries (arrows) from the internal carotid artery. C, Contrast-enhanced 3D TFE image demonstrates a well-enhanced mass lesion at the right cavernous sinus and posterior cranial fossa. D, Conventional ASL image shows a tumor vascular territory with higher perfusion than that in the normal-appearing cortex (arrow). The degree of tumor perfusion is classified as grade 3. E, RPI acquired at the same level as D. The hypervascular territory is not depicted on the RPI (arrow). The extent of tumor perfusion on the 2 techniques is classified as completely different.
Discussion
RPI was able to provide functional information on the tumor vascular territory and the feeding arteries. Because RPI has partial labeling of the proximal arterial tree, the labeling efficiency for RPI is lower than that for conventional ASL imaging. We performed RPI with the advantage of long T1-weighted relaxation times and high signal intensity–to-noise ratios due to 3T. Therefore, this advantage to RPI may have contributed to our results. The overall quality of RPI was considered sufficient for all 6 supratentorial tumors; the image quality of the infratentorial tumor or the tumor near the skull base was judged as fair. Although susceptibility artifacts in the posterior fossa and regions near the skull base may affect the quality of RPI, it was possible to assess tumor vascularity in this study.
In the 4 angiographically confirmed meningiomas to which the dominant supply was the external carotid artery, the extent of the vascular tumor territory visualized on RPI coincided with or was partially different from that depicted on conventional ASL images. On the other hand, in 1 meningioma in which the vascular supply to the tumor was the internal carotid and ophthalmic arteries, the territories on conventional ASL and RPI images were completely different. This suggests that RPI with selective labeling of the external carotid artery can accurately depict vascular supply from the external carotid artery in extra-axial brain tumors.
In the 2 angiographically confirmed cases in which the extent of the vascular tumor territory on RPI and conventional ASL images was scored as partially different, the territory appeared smaller on RPI than on conventional ASL images. On the basis of our DSA findings, we think that this observation is attributable to parasitic or meningeal vascular supply from the internal carotid or vertebrobasilar artery territories. When the extent of the tumor vascular territory on RPI is completely different from that on conventional ASL images, feeders other than the external carotid artery should be considered.
Although we did not assess the clinical value of this technique in this study, some clinical applications of RPI were considered in the evaluation of brain tumors. First, RPI may assist in differentiating intra- and extra-axial tumors. The distinction on conventional MR imaging alone is sometimes difficult. Because RPI may help to identify the arteries that feed the tumor, it may be useful in this situation. Second, RPI may provide information about the feeding arteries of extra-axial tumors before preoperative embolization. In patients with hypervascular extra-axial brain tumors (ie, meningiomas), embolization of the tumors is useful as a preoperative adjuvant therapy in mitigating blood loss during surgical resection.12,13 However, embolization is not applied when the dominant supply is clearly from the internal carotid artery. This information might be obtained by using the RPI technique. Third, intra-arterial contrast-injection CT and MR imaging can provide volumetric information of both the tumor and the distribution of vascular territories related to embolization.14,15 RPI might reveal similar information without catheterization. Further clinical investigation by using RPI and DSA or intra-arterial contrast-injection CT and MR imaging is needed to clarify the usefulness for these applications.
Our study has some limitations. First, although DSA is the criterion standard for evaluating the vasculature of extra-axial brain tumors, 3 patients whose tumor feeding arteries were identified on MRA did not undergo DSA because cerebral angiographic complications remained. Second, manual selective labeling of the external carotid artery may have affected the RPI results. We performed RPI by placing selective labeling slabs over the external carotid artery by referring to MRA studies. Because the external carotid artery and the internal carotid artery run in different directions, we were able to place the labeling slabs without overlaps with the internal carotid artery. Therefore, we think that the RPI scans depicted only the vascular supply from the external carotid artery. Third, we did not directly compare RPI of the external carotid artery with RPI of the internal carotid artery. Because the internal carotid and common carotid arteries continue in a similar direction, the labeling slab of RPI cannot be placed for only the internal carotid artery. Therefore, we were not able to perform the selective labeling of the internal carotid artery in this study. Fourth, we did not perform a quantitative assessment of tumor perfusion, though the quantification of perfusion may be useful in the preoperative evaluation of brain tumors.
In conclusion, RPI with selective labeling of the external carotid artery was feasible for assessing the vascular supply of hypervascular extra-axial brain tumors. In the patients who underwent DSA, RPI findings correlated closely with the vascular supplies seen during selective intra-arterial DSA. Because RPI does not involve ionizing radiation or exogenous contrast media injection, it is expected that this noninvasive technique may supplement or replace x-ray angiography in certain clinical situations. Superselective labeling of vessels such as the internal carotid artery or ophthalmic artery on RPI would render this technique highly useful for the detailed evaluation of brain tumor feeders. Further studies on larger populations are necessary to clarify the potential role of RPI in patients with extra-axial brain tumors.
References
- Received May 5, 2009.
- Accepted after revision July 23, 2009.
- Copyright © American Society of Neuroradiology