Abstract
BACKGROUND AND PURPOSE: An infrequent occurrence during endovascular treatment is protusion of detachable coils into the parent lumen with a subsequent thrombosis within in the parent vessel or embolic events. We report the short- and intermediate-term angiographic and clinical outcomes of patients who experience coil or loop protrusions and are managed with medical or additional endovascular treatments.
MATERIALS AND METHODS: The coil protrusions were identified by retrospective review of 256 consecutive patients treated at 3 centers with endovascular embolizations for intracranial aneurysms and subsequently categorized as grade I when a single loop or coil protruded into the parent vessel lumen less than half the parent artery diameter; grades II and III were assigned when a single coil or loop protruded more than half the parent artery diameter, respectively.
RESULTS: There were 19 patients with grade I (n = 9), grade II (n = 4), or grade III (n = 6) coil protrusions. Patients with active hemodynamic compromise (n = 6) had intracranial stents placed in addition to aspirin (indefinitely) and clopidogrel (range, 1–12 months; mean, 4.5 months) treatment. The remaining patients were placed on aspirin indefinitely. Complete aneurysm obliteration was achieved in all patients except in 3 in whom near-complete obliteration was achieved. Two patients had intraprocedural aneurysm ruptures, both of whom survived hospitalization. There were 4 deaths (4–21 days), all due to major strokes in different vascular distributions related to vasospasm (unrelated to the coil protrusion).
CONCLUSIONS: Management of coil protrusions with antiplatelet therapy and placement of stents (in selected patients) appears efficacious in preventing vessel thrombosis.
Abbreviations
- ACA
- anterior cerebral artery
- AcomA
- anterior communicating artery
- GDC
- Guglielmi detachable coil
- HH
- Hunt and Hess
- IA
- intra-arterial
- ICA
- internal carotid artery
- ICH
- intracerebral hemorrhage
- IV
- intravenous
- mRS
- modified Rankin Scale
- PcomA
- posterior communicating artery
- SAH
- subarachnoid hemorrhage
- TIMI
- Thrombolysis in Myocardial Infarction
Endovascular treatment of intracranial aneurysms is a widely accepted alternative to surgical treatment for certain aneurysms.1 An infrequent occurrence during endovascular treatment is protrusion of detachable coils into the parent lumen with a subsequent thrombosis within the parent vessel or embolic events.2–4 Overall, thromboembolic complications occur in 2.5%–28% of cases with a variable and often uncategorized component related to coil protrusion.6,7 Coil protrusion may result from size mismatch between the coils and aneurysm, inadequate position of the microcatheter within the aneurysm, and/or wide-neck aneurysms.4,5
Several techniques for managing coil protrusions into parent vessels have been described in case reports, including removal and repositioning of the coil before electrolytic detachment,3 removal of an already-detached coil by using a snare,2,10–12 repositioning or redirecting the protruding coil by using balloon inflation,8,9,13 or stent reconstruction of the lumen of the parent vessel.3,4,14 We report the short- and intermediate-term angiographic and clinical outcomes of a consecutive series of patients who experienced coil protrusion and were managed with medical or additional endovascular treatments, with emphasis on the magnitude of the protrusion.
Materials and Methods
Patients
We retrospectively reviewed the medical records and angiographic images for all patients who underwent endovascular procedures for intracranial aneurysms at 3 institutions. The patients were identified by using local registries maintained by the cerebrovascular/endovascular programs that track all patients who undergo endovascular treatment. The patients reviewed were treated at the Neurosurgery, Endovascular, and Spine Center, Austin, Texas, between January 2004 and December 2009, and at Hennepin County Medical Center (Minneapolis, Minnesota) or the University of Minnesota Medical Center (Minneapolis, Minnesota) from September 2006 to December 2009. The protocol for collecting data was reviewed and approved by the institutional review boards at institutions that required such approvals for retrospective studies.
We identified patients who had coil protrusions by reviewing the procedural notes of all endovascular procedures performed in those institutions and confirmed them by review of pre-, intra-, and postprocedural angiograms. The following information was collected from the medical records of the patients with coil protrusions: presenting symptoms (SAH, asymptomatic, or other); procedural details with emphasis on factors associated with protrusion; immediate clinical and/or angiographic consequences and treatment used; immediate postprocedural outcomes; and outcomes at follow-up with emphasis on recurrent or new ischemic events or the need for further treatments. Additional procedural aspects recorded included aneurysm location and number and type of coils used. Details of any periprocedural neurologic deficits or bleeding complications were also recorded. Recurrent strokes were classified as either minor with mRS scores of ≤2 or major with mRS scores of >2 at discharge following the primary procedure and at follow-up.
Protocol for Coil Embolization
The endovascular procedures were performed predominantly with the patients under general anesthesia and systemic heparinization. Infrequently, the procedures were performed under awake conditions. Using a standard percutaneous approach, we placed a 6F introducer sheath in the femoral and, infrequently, in the radial arteries. A 5F or 6F guide catheter, most frequently an Envoy guide catheter (Cordis, Miami Lakes, Florida), was advanced over the guidewire under fluoroscopic guidance. Using road-mapping techniques, we advanced the catheter into the internal carotid or vertebral artery. An IV heparin bolus (30–50 U/kg) was administered either at the time of guide-catheter placement or immediately after placement of the first detachable coil to achieve an activated coagulation time above 250 seconds. Digital subtraction angiographic images were acquired to identify the best projections that allowed visualization of the parent artery and aneurysm junction and measurement of pertinent dimensions of the aneurysms before coil placement. Microcatheters (Excelsior SL-10 or Excelsior 18; Boston Scientific, Natick, Massachusetts) were advanced over a microwire (Synchro 0.014-inch or Transend 0.014-inch, Boston Scientific) into the aneurysmal sac. Detachable coils were delivered into the aneurysmal sac and deployed by using standard techniques. In addition to GDCs (Boston Scientific), several other detachable coils with unique attributes, including Matrix (Boston Scientific), Trufill DCS Orbit (Cordis), HydroCoil (MicroVention Terumo, Aliso Viejo, California), and MicroCoil (Micrus Endovascular, San Jose, California), were used as deemed necessary. The procedure was continued until angiographic obliteration was complete, no further coils could be placed, and/or complications occurred.
In the event of coil protrusion and active hemodynamic alteration or pulsating coil segment, various techniques were used by the operators, including self-expandable intracranial stent deployments. When the decision was made to place a self-expanding stent, the microcatheter was withdrawn from the aneurysmal sac and advanced past the ostium of the aneurysm into the distal arterial segment by using a microguidewire. Then a self-expandable intracranial stent (Enterprise, Cordis or Neuroform, Boston Scientific) was deployed across the aneurysm neck either through the microcatheter or through the delivery catheter after exchange over a microwire. The intent of stent placement was to push back the protruded coil or loop into the aneurysm sac or trap the protruding mass between the wall of the parent artery and the stent.
Postdischarge Outcome
Clinical outcome and coil protrusion stability were determined in patients who were discharged from the hospital. Information was acquired by reviewing clinical notes, follow-up angiograms, medical records from any rehospitalizations, and telephone interviews by 1 of the investigators. Details obtained included any recurrent strokes, functional status defined by mRS, and the causes of mortality (if indicated). The occurrence of a new or recurrent stroke or death was recorded along with the time interval between the initial procedure and the new event. Recurrent stroke was defined as a new neurologic deficit consistent with the definitions for ischemic or hemorrhagic stroke, occurring after a period of unequivocal neurologic stability or improvement lasting for at least 24 hours and not attributable to edema, mass effect, brain shift syndrome, or hemorrhagic transformation of the incident ischemic event.15 If a new event occurred in the distribution of the treated aneurysm, the event was classified as “definitely related” to coil protrusion; if another explanation was identified, it was referred to as “probably-related”; and if another explanation was identified such as moderate to severe vasospasm or if the event occurred in another vascular distribution, it was classified an “unrelated.”
Image Analysis
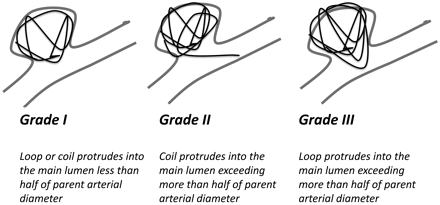
Angiographic images of the embolized aneurysms were acquired from the PACS and reviewed by 2 investigators. All images from the different health systems were reviewed centrally by 2 investigators after the data were de-identified. Pre-, intra-, and postprocedural angiograms were reviewed to confirm and categorize the angiographic severity of coil protrusion. The severity was graded by using a 3-point scheme. A schematic representation of the various grades of the scheme is shown in Fig 1. If a loop or coil protruded into the parent vessel lumen less than half the parent artery diameter, it was given a grade I. When either a coil or loop protruded in the parent vessel lumen more than half of the parent artery diameter, it was given a grade II or III, respectively.
Grade I, Loop or coil protrudes into the main lumen less than half of parent artery diameter. Grade II, Coil protrudes into the main lumen exceeding more than half of parent artery diameter. Grade III, Loop protrudes into the main lumen exceeding more than half of parent artery diameter.
Results
Patient Population
A total of 256 patients underwent 266 endovascular treatments for intracranial aneurysms in our institutions during that study period. Of those 266 procedures, 19 procedures in 19 patients were complicated with coil protrusions (7% of procedures). The mean age of study subjects was 47.3 ± 9.3 years (range, 33–72 years); 14 were women. The indication for the coil embolizations was ruptured intracranial aneurysm in 18 of the 19 patients. One patient had an unruptured intracranial aneurysm detected by MR angiography during screening on the basis of a family history of aneurysms. The demographic and clinical data, including patient age, sex, presenting SAH scale, aneurysm location and size, and intervention performed, are summarized in the On-line Table.
Technical Aspects
The aneurysms were located in the paraophthalmic segment (n = 3), supraclinoid segment (n = 2), bifurcation of the ICA (n = 2), AcomA (n = 7), ACA (n = 2), pericallosal artery (n = 1), PcomA (n = 1), and basilar artery (n = 1). There were 9 patients with grade I, 4 with grade II, and 6 with grade III coil/loop protrusions. Figures 2 and 3 show examples of various grades of coil protrusions. There were 10 patients with major coil/loop protrusions (grades II and III) and 9 with minor coil/loop protrusions (grade I). All the events were either single coil or loop protrusions; no multiple coil or loop protrusions were observed. All events occurred during the embolization procedure except 1 protrusion that was detected by angiography performed 4 days after the procedure.
Serial angiographic images of a 48-year-old man who underwent endovascular treatment for a ruptured left ICA terminus aneurysm. A, Immediate postprocedural image demonstrates a grade I protrusion. B, Six-month follow-up corresponding image demonstrates spontaneous resolution of the coil protrusion.
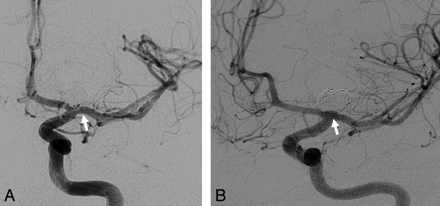
Serial angiographic images of a 40-year-old woman who underwent endovascular treatment for a ruptured paraophthalmic segment ICA aneurysm. A, Immediate postprocedure image demonstrates a grade II coil protrusion into the parent vessel lumen. B, At 18-month follow-up, angiographic images demonstrate persistent but unchanged grade II coil protrusion.
Of the 10 patients with grade II or III protrusions, 5 protrusions were associated with hemodynamic alterations evident by delay in contrast opacification in the parent vessel adjacent or distal to the segment with protrusion. Coil repositioning within the aneurysm was attempted multiple times after the coil protrusions. However, in all 19 patients, the coil protrusions persisted. Balloons were inflated in front of the aneurysm neck in 2 patients in an unsuccessful attempt to reposition the protruding coil into the aneurysm. Two of the 19 procedures were complicated by intraprocedural aneurysm ruptures that occurred concurrently with the coil/loop protrusions. These episodes were managed by reversal of anticoagulation with protamine and intracranial pressure control with mannitol, hypertonic saline, and emergent ventriculostomy catheter placement. No balloon expansion, intracranial stent, or platelet glycoprotein IIb/IIIa inhibitors were used in either of the 2 patients.
On admission, all the patients except 1 with an unruptured aneurysm were started on hypertonic saline infusion to control intracranial pressures. Moreover, 16 of the 18 patients who presented with SAH required ventriculostomy catheters to be placed to monitor and control intracranial pressures.
Platelet Glycoprotein IIb/IIIa Inhibitors
Intravascular thrombus formation during the embolization procedure, diagnosed by an angiographic filling defect with occlusion of the parent vessel, occurred in 2 patients (patients 6 and 7), both of whom were undergoing embolization of an AcomA aneurysm. There was no coil or loop protrusion in patient 6 when the thrombus formed. The TIMI scale was used to measure the angiographic severity of the occlusion and response to treatment.16,17 Both patients had a TIMI grade of 0 at the time of thrombus formation. In patient 6, a 2-mg bolus of abciximab (0.2 mg/mL) was administered IA through a microcatheter placed next to the thrombus for 20 minutes. After the administration of abciximab, there was complete resolution of the filling defect with good distal flow (TIMI grade III). A follow-up angiogram obtained the next day demonstrated no new thrombus formation. In patient 7, a bolus of eptifibatide, 10 mg (143 μg/kg), was administered IA through the microcatheter placed within the thrombus. After 15 minutes, the patient had some flow through the thrombus but no distal flow was observed (TIMI grade I). The patient was then placed on continuous IV infusion of eptifibatide, 41 mcg/min for 24 hours. A follow-up angiogram obtained the next day demonstrated resolution of thrombus with improvement in flow in some branches (TIMI grade II) with concurrent cerebral vasospasm in multiple arteries. Due to poor baseline neurologic status, no definite neurologic change could be detected. The eptifibatide infusion was continued for another 2 hours, and clopidogrel, 300-mg bolus, was administered. The patient was found to have large and small infarctions in middle cerebral artery and ACA distributions, respectively, on follow-up CT 3 days after the procedure. Further angiographic follow-up was not performed. Another patient was treated with eptifibatide, 8.7-mg IV bolus, administered as a prophylactic measure without evidence of filling defect or poor distal flow.
Intravascular Stent Placements
Six patients underwent stent placement within the same procedure after coil protrusion was detected. No antiplatelet therapy was administered before the stent deployment in any of the 6 patients. One patient with a grade I coil protrusion pulsating in the parent vessel underwent self-expandable intracranial stent deployment (Neuroform, Boston Scientific). Five patients with grade II or III protrusions and active hemodynamic alteration underwent self-expandable intracranial stent deployment (On-line Table): 4 Neuroform stents (Boston Scientific) and 1 Enterprise stent (Cordis) were used, followed by antiplatelet therapy. The coil embolization procedure was resumed after the stent deployment only in patient 9.
Nonparenteral Antiplatelet Agents
Eighteen of the 19 patients were given aspirin, 325 mg orally or 300 mg as a suppository after coil or loop protrusions were detected, in the angiographic suite. Aspirin was continued as a 325-mg daily dose. One patient did not receive any antiplatelet therapy. Except for 2 patients, aspirin was continued indefinitely for the surviving patients; in those 2 patients, the aspirin was discontinued after the first month. The dose of aspirin was reduced to 81 mg/day after 1 month in 2 other patients. The 6 patients who received intracranial stents were placed on clopidogrel, 75 mg for 1–12 months (mean, 4.5 months), in addition to the aspirin indefinitely. No symptomatic ICH was detected during the period of dual antiplatelet treatment. Because the stents were placed in the acute setting of SAH, the patients were not pretreated with antiplatelet therapy.
Immediate Angiographic Results
The final results of the embolization procedure were categorized as complete, near-complete, and partial obliteration on the basis of existing definitions.23 Three patients had small residual filling of the aneurysm neck (near-complete) at the end of the embolization procedure (patients 1, 3, and 4). Patient 1 required additional coil embolization of the residual aneurysm neck 2 days later. Follow-up angiography in patient 3 five months later demonstrated spontaneous obliteration of the residual aneurysm neck. Patient 4 did not undergo follow-up angiography in our institutions because he had moved to another state after discharge. The other 16 patients had complete aneurysm obliteration after the embolization procedure.
In-Hospital Outcomes
There were 4 patients who died within the 1-month period after endovascular treatment. Two patients had grade I and 2 had grade III protrusions. All 4 patients (patients 5, 6, 7, and 12) developed new major ischemic strokes involving multiple distributions. Two of those 4 patients had an initial HH grade of 5 and the other 2 had an HH grade of 3. The mean time intervals between coil embolization procedure and detection of a new infarct and death were 4 and 9 days, respectively. Infarctions involving the distribution of the artery with coil protrusion were only noted in patient 7, as mentioned above. Severe vasospasm was the predominant finding on follow-up angiograms obtained after the development of new deficits, which was considered “unrelated” to the coil/loop protrusions in all the cases. None of the aneurysms in these 4 patients had re-ruptured during the coil embolization procedure. On the other hand, neither of the 2 patients who had intraprocedural rupture developed vasospasm-related new ischemic strokes (On-line Table). Of the 6 patients who underwent stent placement, none had an ischemic stroke in the distribution of the stent placement. The vasospasms were treated with multiple angioplasties and/or IA vasodilator infusion procedures in 3 patients (patients 5, 6, and 7). No further endovascular treatment was undertaken in patient 12 because she had developed multiorgan failure in addition to the major stroke. One patient (patient 1) was found to have a small asymptomatic ICH on a routine CT. She was on IV heparin infusion for treatment of deep venous thrombosis. The heparin was discontinued, and an inferior vena cava filter was placed. She was started on subcutaneous enoxaparin 5 days later at the time of discharge to a rehabilitation facility and continued it for 6 months.
Posthospital Outcomes
The surviving 15 patients were followed up for a mean period of 13.8 months (range, 3–48 months). One patient died from progression of metastatic breast cancer 5 months after the procedure. None of other 14 patients had residual disability (mRS score of >2) at last available follow-up. A total of 13 patients underwent a follow-up angiography after a mean period of 12.8 months (range, 3–48 months). There was no coil compaction or aneurysm regrowth. In 2 patients, the coil protrusion had spontaneously resolved. In the other 11 patients, no angiographic thrombosis or progression of the coil protrusion (change in grades) was observed.
Discussion
Coil protrusion has clinical significance because the interaction of the protruded mass and flow abnormalities can invoke thrombogenic responses or the coil mass may itself become an embolus. When blood first contacts a foreign surface like coils, a sequence of events is initiated that often ends in blood coagulation and thrombus formation. Initially, a thin layer of platelets and fibrinogen covers the surface of the coils. The magnitude of the initial reaction depends on the surface charge, chemical properties, topographic features of the coils, and the pattern of blood flow in the vicinity.18 In this study, the rate of major complication or death related to the SAH was 21% (4/19). There was only 1 case with a small distal thrombi noted in the last angiogram. However, the cause of death was thought to be due to severe vasospasm in all 4 patients. Those 4 had high initial HH scores and were at high risk for adverse outcome.
In addition, 2 of the 19 (10.5%) patients had intraprocedural aneurysm ruptures; both survived the hospitalization (On-line Table). The rate in the current study was higher than the 1.4%–2.6% reported in other larger studies.19,20 This observation suggests that coil prolapse may be associated with increased vulnerability to aneurysmal rupture. It remains unclear whether the vulnerability is related to aneurysm characteristics or directly associated with the prolapse. The small number of patients with coil protrusions (7% of our total procedures) prevents a more in-depth analysis of the factors that affect intraprocedural aneurysm rupture. Previous studies have identified recent aneurysmal rupture, smaller aneurysms, AcomA or posterior circulation location, and aneurysms with associated teats are at increased risk of intraprocedural rupture.20 Coil protrusions spontaneously resolved in 2 patients on follow-up angiograms at 4 and 6 months post-treatment. The spontaneous resolution was thought to be due to coil compaction resulting from the complex interaction between the local hemodynamics and intra-aneurysmal flow, resulting in significant forces on the coil mass.25
Antiplatelet Therapy
In this series, 18 of the 19 patients were given aspirin immediately after their coil protrusion. The 6 patients with intracranial stent placement due to active hemodynamic compromise were placed on clopidogrel, 75 mg, for 1–12 months (mean, 4.5 months), in addition to aspirin indefinitely. The optimal antiplatelet regimen and duration are not well-understood. In a review of 23 studies using GDCs for treatment of intracranial aneurysms of the anterior or posterior circulation, the rate of thromboembolic events was lower among patients treated concurrently with aspirin (28 events among 435 patients, 6.4%), compared with patients not treated with aspirin in the perioperative period (99 events among 112 patients, 8.9%).21 Most authors recommend several months of dual antiplatelet therapy, ranging from a few months to life-long treatment for patients with coil protrusions with or without stent placement. Luo et al4 administered both aspirin and clopidogrel for 3–6 months after stent placement in 9 patients with large coil protrusions.4
Endovascular Interventions
Microsnares (Amplatz, White Bear Lake, Minnesota) have been used to retrieve coil or loop protrusions and associated thromboembolism.2,10,11 Dinc et al2 acknowledged that this technique requires the microsnare to be positioned distal to the migrated coil and the expanded loop to be placed circumferentially across the protruded coils adhering to the parent artery wall. Such positioning may not always be possible. In addition, snaring protruded coils or loops can cause adverse outcomes such as embolic migration of the coils, rupture, or dissection.2,8,12 The remodeling or rescue balloon technique has been described by some authors as useful in managing coil protrusions, particularly in wide-neck aneurysms.8,9,13 This is a technique in which a nondetachable balloon is inflated in front of the neck of the aneurysm, predominantly during replacement of coils.11 The use of balloon inflation to reposition deployed coils is less likely to achieve success, as evident in 2 of our patients. Some of the risks of this technique include rupture of the aneurysm or parent vessel, balloon-related thrombus, and occlusion of adjacent perforating arteries.8,13
Stents have similarly been used to manage coil protrusions by reconstructing the parent artery lumen,3,4,14 either by pushing back the protruded coil or loop into the aneurysm sac or by trapping the protruding mass between the wall of the parent artery and stent.4 Luo et al4 had used stents to reconstruct the parent artery lumen in their series of 9 patients; 8 of the 9 patients had large coil protrusions without hemodynamic compromise, and 1 had hemodynamic compromise in the parent vessel. In our series, stents were placed in only 6 of the 19 patients who had coil protrusions pulsating in the parent vessel or causing hemodynamic compromise. We found that stent placement was effective in patients with grades II and III protrusions but not necessarily in those with grade I protrusions.
Although the different interventions to treat coil protrusions have been discussed by various investigators in anecdotal reports,2–4,8–14 we provide a comprehensive review of consecutive patients with coil prolapse and outcomes following various strategies. Our review suggests that most small coil protrusions are asymptomatic and can be managed with antiplatelet therapy alone. Larger coil protrusions that comprise more than half the parent artery diameter or cause hemodynamic alteration require endovascular intervention in addition to antiplatelet therapy. The grading scheme we propose is an easy and practical method to categorize the magnitude of protrusions on the basis of angiographic images. All grade I protrusions are non-flow-limiting. Whereas both grade II and III protrusions can be potentially flow-limiting, other factors such as angulation and diameter of parent artery and other flow-limiting factors such as proximal spasm or dissection also contribute to hemodynamic effects of prolapse. We developed a proposed scenario-specific management strategy for coil protrusions and the presence of hemodynamic compromise and/or intravascular thrombosis as presented in the On-line Figure.
Our study was predominantly a reflection of procedures performed by using bare platinum coils; the adverse event rates may be higher if a higher proportion of procedures were performed by using bioactive coils. However, the HydroCoil for Endovascular Aneurysm Occlusion trial found that HydroCoils (MicroVention) had thromboembolic complications similar to those of the platinum-based coils, 8.1% versus 8.2%, respectively.22
Limitations
In this retrospective review, we relied on medical documentation to identify cases of coil protrusion; however, such ascertainment methodology may have missed protrusions of lesser magnitude. In addition, this was a relatively a small series with intermediate-term follow-up. We were also unable to provide an estimate of prevalence and consequences of “pseudoprolapse,” which is the appearance of prolapse despite the coils being within the aneurysm sac due to overlap of the aneurysm sac with the parent artery. The treatment strategies were selected by the treating physician; this practice led to some variations in management. We observed a very low rate of complications associated with stent placement in our series. The small number of patients with SAH who underwent stent placement (n = 6) precludes any definitive comments regarding the rate of complications associated with stent placement in the acute setting of SAH. Early deaths due to other concurrent conditions such as vasospasm prevented intermediate-term assessment in some patients.
Conclusions
Delayed thrombosis or progression of coil protrusion appears to be uncommon after antiplatelet treatment alone or in combination with self-expanding stents. Intravascular stent placement appears to be an effective option if treatment is required due to hemodynamic compromise.
Footnotes

Indicates article with supplemental on-line table.
Indicates article with supplemental on-line photos at www.ajnr.org.
Disclosures: M. Fareed and K. Suri, Research Support (including provision of equipment or materials): NIH, Details: NIH-K1 award provides 75% salary support. A. Qureshi, Research Support (including provision of equipment or materials): American Hospital Association, NIH, Details: grants.
References
- Received September 14, 2010.
- Accepted after revision December 26, 2010.
- © 2011 by American Journal of Neuroradiology