Abstract
SUMMARY: Looking for anomalies distributed in DMV territory, we reviewed 78 fetal MR imaging examinations performed at our institution reporting unequivocal cerebral clastic lesions. We selected 3 cases, all of which had severe cardiocirculatory failure and parenchymal frontoparietal WM hemorrhagic lesions with characteristic fan-shaped distribution. Brain edema and other signs of venous hypertension were also evident. Our data suggest that in utero transient venous hypertension may be responsible for the onset of atypical frontal-located PVL.
Abbreviations
- ADC
- apparent diffusion coefficient
- DMV
- deep medullary veins
- PVL
- periventricular leukomalacia
- WM
- white matter
Deep medullary veins have been noted to play a role in the development of frontoparietal hemorrhagic PVL in premature and term neonates.1,2 Etiology and time of onset of these lesions are often unknown. Because the deep vein drainage system is relatively immature in the developing brain,1 we investigated whether pathologic changes within DMV (such as congestion, microhemorrhage, perivenous WM signal intensity, and ADC abnormalities) could be associated with clastic lesions in utero, detected by fetal MR imaging. Three pediatric neuroradiologists (A.R., C.P., and C.D.) reviewed all fetal MR imaging reports from our institution that mentioned destructive lesions, between 2000 and 2009, to select those probably due to DMV drainage impairment. Lesions were examined for distribution, morphology, signal intensity, and other associated cerebral MR imaging findings. Specifically, we selected those cases that reported focal T2 hypointense wedge-/fan-shaped periventricular WM lesions with distribution in DMV territory. Cases with extensive parenchymal disruption or cortical-subcortical lesions possibly distributed in specific arterial territories were excluded. Examinations were performed for clinical purposes according to the informed consent form, and this study complied with research guidelines for clinical review studies of our institution.
Case Reports
MR imaging was performed at 1.5T (with a cardiac phased-array coil) by using multiplanar single-shot fast spin-echo T2-weighted sequences (TR, 3000 ms; TE, 180 ms; FOV, 320 mm; matrix, 256 × 256; section thickness, 3–4 mm). T1-weighted (TR, 300 ms; TE, 14 ms; FOV, 340 mm; matrix, 256 × 256; section thickness, 5.5 mm; acquisition time, 14 seconds during maternal apnea) and diffusion-weighted (b-value, 0 and 600 s/mm2; TR, 1000 ms; TE, 90 ms; FOV, 340 mm; matrix, 256 × 256; section thickness, 5.5 mm; acquisition time, 14 seconds during maternal apnea) images were also acquired. ADC maps were calculated off-line.
Patient 1
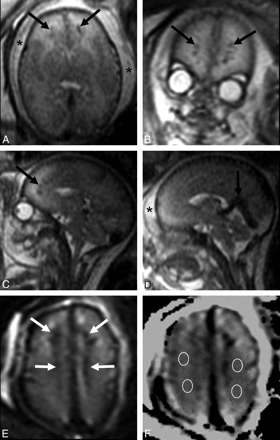
A 27-year-old woman carrying a male fetus affected by severe cardiomyopathy presented with oligohydramnios and hydrops due to cardiocirculatory failure on sonography performed at 31 weeks' gestation. MR imaging on the same day demonstrated severe brain swelling and reduction of cortical and ventricular spaces. T2-weighted signal-hyperintensity of bihemispheric WM with blurring of parenchymal layering was also present. Small linear fan-shaped T2 hypointense and T1 hyperintense anomalies were detected in the frontal periventricular WM. Lesion hypointensity on T2* b = 0 images suggested perivenous microhemorrhage. ADC in the WM around focal lesions was high (2.3 μm2/s), confirming the presence of interstitial edema. Enlargement of the main venous collectors was detected (Fig 1). The fetus died at 33 weeks' gestation; postmortem examination confirmed subcutaneous edema, hydrothorax, ascites, hepatic, renal and suprarenal hemorrhages, leptomeningeal congestion, and cerebral parenchymal thinning with wide bilateral frontal WM ischemic areas.
Patient 1. A−C, Axial, coronal, and sagittal single-shot fast spin-echo T2-weighted images show diffuse brain swelling, with size reduction of cortical and ventricular spaces and slight diffuse hyperintensity of the WM. Small linear fan-shaped hypointense lesions are also detected in the frontal WM (black arrows). Subcutaneous edema is also seen (asterisks). D, Sagittal single-shot fast spin-echo T2-weighted image confirms soft-tissue thickening (asterisk) and shows vein of Galen and straight sinus enlargement (black arrow). E, Frontal periventricular lesions (white arrows) are hypointense on a T2* b = 0 image, suggesting the presence of microhemorrhage. F, ADC map at the level of the centrum semiovale with regions of interest positioned in the perilesional WM.
Patient 2
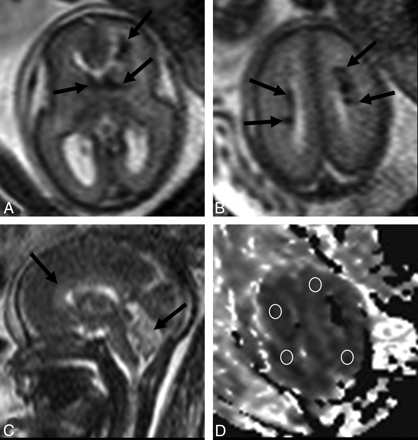
A 33-year-old woman was referred for fetal MR imaging at 23 weeks' gestation for a parvovirus infection. The fetus underwent blood transfusion 1 week before, because of severe anemia. Ultrasonography demonstrated hydrops and periventricular hyperechogenicity. MR imaging confirmed subcutaneous edema and ascites; abnormal brain features consisted of T2-weighted signal-hypointensity of ventricular margins and fan-shaped periventricular frontoparietal T2 hypointense and T1 hyperintense WM alterations (Fig 2). Although cortical and ventricular spaces appeared normal, interstitial edema was suspected because of abnormally high ADC (2.3 μm2/s) in the WM around focal lesions. The fetus died 2 days later; brain pathology could not be performed because of tissue lysis.
Patient 2. A, Coronal single-shot fast spin-echo T2-weighted image demonstrates a wedge-shaped pattern of periventricular lesions (arrows); head subcutaneous tissue thickening is also evident (asterisk). B, Coronal T1-weighted image shows hyperintensity of periventricular lesions (arrows). C, Sagittal single-shot fast spin-echo T2-weighted image confirms subcutaneous edema (asterisk) and better demonstrates the anterior distribution of the lesions (arrows). D, ADC map with regions of interest positioned in the subcortical WM.
Patient 3
A 34-year-old woman with a monochorionic twin pregnancy, complicated by pulmonary valve stenoinsufficiency in both fetuses, underwent sonographic examination a few days after the death of a co-twin at 24 weeks' gestation. Sonography on the survivor showed ascites and signs of anemia on cerebral Doppler sonography. MR imaging on the same day demonstrated severe cerebral and cerebellar swelling. Linear radial-oriented T2 hypointense lesions were detected in the frontal periventricular WM and the deep centrum semiovale bilaterally. ADC in the centrum semiovale was remarkably reduced (0.6 μm2/s), suggesting cytotoxic edema and acute damage. Head and neck subcutaneous tissue thickening was visible (Fig 3). The fetus died a few hours later; brain pathology could not be performed because of tissue lysis.
Patient 3. A, Axial single-shot fast spin-echo T2-weighted image at the level of the basal ganglia shows hypointense wedge-shaped lesions anterior to the left frontal horn and small linear lesions converging on the ventricle angle bilaterally (arrows), in addition to diffuse brain swelling with size reduction of cortical spaces. B, Axial single-shot fast spin-echo T2-weighted image at the level of centrum semiovale shows diffuse brain edema and periventricular frontoparietal hypointense radial-oriented lesions (arrows). C, Median sagittal single-shot fast spin-echo T2-weighted image shows slight hyperintensity and swelling of the corpus callosum and cerebellar vermis (black arrows). D, ADC map at the level of the centrum semiovale with regions of interest positioned in the perilesional WM.
Discussion
The reported cases show how fetal cardiac failure may be associated with DMV congestion, perivenous hemorrhage, and various patterns of brain edema. The peculiar features of such intracranial alterations may be related to the relatively immature fetal venous parenchymal system.1
Medullary veins draining from cerebral WM are divided into superficial and deep groups. The DMV group consists of relatively long venous channels originating in the subcortical WM, running centripetally toward the ventricular surface and draining via the subependymal veins. DMVs reach the ventricular wall, becoming large and fewer in number and, finally, converging at specific points (mainly the lateral corner of the ventricles) with a typical fan-shaped pattern. These points are localized along a line that runs above the caudate nucleus and, posteriorly, bifurcates along its tail and the roof of the occipital horn.3 Some authors described a third group of medullary veins (the intracerebral anastomotic or transcerebral veins), which cross the entire hemisphere, reaching the subependymal veins.4,5 Normal DMVs are difficult to identify on MR imaging, and few reports of their pathologic aspects have been reported in neonates.6 Our 3 cases had parenchymal WM lesions with distribution in the DMV territory in addition to cytotoxic or interstitial-vasogenic brain edema and other cerebral and extracerebral signs of venous hypertension. It is known that generalized brain swelling could be part of a near-death condition; however, regarding our 3 cases, fetal death was more likely due to cardiac failure rather than to brain stem herniation or compression. Lesions were always located in the frontoparietal periventricular WM; no lesions were identified in the temporo-occipital WM, probably because of the possible higher frequency of transcerebral medullary veins in these areas, which might represent a protective factor. Intraparenchymal hemorrhage had been previously described in association with PVL as the result of bleeding into tissue already damaged by ischemia.7 In our 3 cases, lesions were partially hemorrhagic, with characteristic wedge-shaped distribution, suggesting the presence of small venous infarctions.
A recent report on fetal cases with severe congenital heart defects showed that diffusivity in the periatrial WM and thalamus was higher compared with that in controls, suggesting that brain development is affected in utero, probably because of cerebral perfusion changes.8 In our 3 cases, all having congenital or acquired cardiopathy, ADC in bihemispheric WM was also abnormal (increased in 2 cases and reduced in 1 case). However, it is not possible to establish how much of this change is due to cardiopathy itself, and how much, to the consequent severe cardiac failure, which, nevertheless, seemed to be the main factor. Our cases are probably very different from the large cohort of fetuses with congenital cardiac anomalies recently presented by Limperopoulos at al,9 which showed significant reduction of brain volume growth with respect to controls. In such a chronic scenario, in which heart failure and central venous congestion were probably not present, the mechanisms producing brain development impairment should have been different from our DMV-associated ones, resulting from a condition of prolonged hypoperfusion and oxygen/glucose deprivation.
The fetal brain is known to be particularly susceptible to hypoperfusion, oxidative stress, and consequent hypoxic-ischemic injury because of arterial immaturity and lack of autoregulation. Such factors can lead to the typical condition of PVL, with WM damage and volume loss mainly affecting the posterior periventricular regions.10 From this limited preliminary series, it appears that DMV could be involved in the development of atypically located PVL (ie, deep frontal lobes) and hemorrhagic necrosis. All of our cases were related to circulatory failure that may cause central venous hypertension. Our cases had severe cardiac failure because intrauterine death occurred some days after imaging. One hypothesis is that venous hypertension could induce DMV engorgement and, hence, small venous infarctions in DMV territory; we suppose that, in some cases, it could be the main factor that leads to leukomalacia. Therefore, hypoxic-ischemic injury might have a role, but, as previously suggested by some authors,6 it is probably not the initial and only event.
References
- Received April 21, 2010.
- Accepted after revision June 3, 2010.
- © 2011 by American Journal of Neuroradiology