Abstract
BACKGROUND AND PURPOSE: PRES-related vasogenic edema is potentially reversible while hemorrhage occurs in only 15.2%–17.3% of patients. However, the true incidence of hemorrhage could be higher when SWI is considered. Thus, we set out to determine the incidence of MH, SAH, and IPH in PRES by using SWI and to particularly evaluate whether such MHs are reversible.
MATERIALS AND METHODS: Thirty-one patients with PRES and SWI were included, 17 having follow-up SWI. Two neuroradiologists reviewed SWI, FLAIR, DWI, and CE-T1WI. The presence and number of MHs (<5 mm) on SWI, SAH, and IPH (>5 mm) were recorded at presentation and follow-up. We evaluated associations between the presence of MH on SWI and DWI lesions, SAH, IPH, contrast enhancement, and MR imaging severity.
RESULTS: Hemorrhage was present in 20/31 patients (64.5%), with MHs on SWI in 18/31 (58.1%) at presentation and in 11/17 (64.7%) at follow-up. SAH was present in 3/31 on SWI and 4/31 on FLAIR, while 2/31 had IPH. At follow-up, no patients had acquired new MHs; 2/5 MHs in 1 patient resolved. Four patients with available SWI before PRES developed MHs after PRES onset. No association was found between the presence of MHs on SWI and DWI, SAH, IPH, enhancement, and MR imaging severity (all P > .05).
CONCLUSIONS: SWI showed a higher rate of MH than previously described, underscoring the potential of SWI in evaluating PRES. Such MHs typically persist and may develop after PRES onset. However, the clinical relevance of MHs in PRES is yet to be determined. We propose that MHs in PRES relate to endothelial cell dysfunction.
ABBREVIATIONS:
- CE
- contrast-enhanced
- INR
- international normalized ratio
- IPH
- intraparenchymal hemorrhage
- MH
- microhemorrhage
- PRES
- posterior reversible encephalopathy syndrome
- T2* GRE
- T2* gradient-recalled echo
PRES is a potentially reversible syndrome, in which CNS involvement may present with a variety of symptoms, the most common being seizure, but PRES also may present with headache, altered mental status, loss of vision, weakness, or loss of consciousness.1,2 PRES was initially described as a “leukoencephalopathy,” being a distinct clinical entity with characteristic imaging findings, in 1996 by Hinchey et al1; the term “leukoencephalopathy” implied deep white matter involvement.2 However, more recent literature has described cortical and subcortical involvement in earlier stages of the disease, where the deep white matter becomes involved by the edema later and in more severe cases.2⇓–4 Notably, this distribution of edema distinguishes PRES from reversible “toxic” leukoencephalopathies, which may be caused by similar factors (such as immunosuppressive and antineoplastic medications), but the pattern of edema in such toxic leukoencephalopathies usually occurs in the reverse fashion beginning with the deep white matter.2⇓⇓–5 The combination of the clinical and imaging findings composing PRES has also been recognized and reported under various other names, such as “hypertensive encephalopathy,” “cyclosporine neurotoxicity,” or “CNS manifestations of toxemia of pregnancy.”6⇓⇓⇓–10
A number of atypical imaging features of PRES have been described, such as the presence of hemorrhage, cytotoxic edema (on DWI), and contrast enhancement; of these, hemorrhage has been reported in larger series to occur in approximately 15%–17% of patients, based on T2*GRE imaging, FLAIR, and CT.2,11 Although PRES-related hemorrhage usually does not affect clinical outcome, larger IPHs may occasionally develop, and these could potentially affect outcome.2,11 However, the frequency of hemorrhage in PRES could be under-reported. SWI is a newer technique that has been shown to be more sensitive than conventional T2*GRE imaging in detecting cerebral MHs in traumatic brain injury and amyloid angiopathy, but it has yet to be proved that SWI has a higher sensitivity for detecting MHs in PRES.12⇓⇓⇓–16 So far, SWI has been used in detecting cerebral MHs whether from cavernomas, amyloid angiopathy, trauma, acute ischemic stroke, or underlying vasculopathy.12⇓⇓⇓⇓–17 In some clinical scenarios, such as trauma, the presence of MH on SWI has not only augmented detection but also has enabled prediction of the clinical prognosis.12,18
Therefore, while SWI has augmented the ability to detect MHs, the incidence of such MHs in PRES has not yet been described. Also, it is as of yet unknown whether such MHs persist after resolution of the neurologic symptoms associated with PRES or whether MHs are present before symptom onset. Thus, we set out to determine the incidence of MHs in PRES and to determine whether they resolve, by retrospectively evaluating the SWI of patients with clinically confirmed PRES. Another goal was to evaluate whether the presence of MHs is associated with the MR imaging severity or whether MHs are associated with other imaging features, such as the presence of: contrast enhancement, cytotoxic edema (based on DWI), IPH, or SAH (sulcal hyperintensity on FLAIR or sulcal hypointensity on SWI).2
Materials and Methods
Patient Selection
For the retrospective selection of the patients, we used software enabling a search throughout the radiology reports within the radiology information system data base (Primordial, Primordial Design, San Mateo, California), via implementing various combinations of search terms such as “PRES,” “posterior reversible encephalopathy,” “hypertensive encephalopathy,” and so forth. The search was limited to MR imaging within a time period following the installation of SWI sequences between September 2008 and September 2010. The criteria for inclusion were similar to those of 2 previous larger studies evaluating hemorrhage in PRES.2,11 These included a clinical diagnosis of PRES based on discharge data and an appropriate clinical basis for PRES based on symptoms of a neurotoxic syndrome (including seizure, mental status change, headache, vision loss, or new weakness) in combination with imaging findings consistent with PRES, including cortical or subcortical involvement on FLAIR images within the posterior frontal, temporal, or parieto-occipital regions.2⇓–4
We chose not to include patients with solely brain stem, basal ganglia, or posterior fossa involvement because such cases do occur but are considered uncommon and our intent was to detect the incidence of MH in regions of PRES-related cortical or subcortical edema; additionally, we did not wish to confound our search for MHs with the presence of normal brain iron in the basal ganglia that may be noted on SWI.19⇓–21 The resulting patients (n = 31) were then submitted for the staff neuroradiologists' review as long as SWI, FLAIR, and DWI were performed, which were standard sequences in all of our brain MR imaging protocols. Additionally, we reviewed each patient's medical record for history of a coagulative disorder, low platelet count (<100,000), high INR (>1.5), and whether the patient was on any anticoagulant medication (including either heparin, warfarin, or enoxaparin), because an increased frequency of PRES has been noticed in patients on anticoagulative medication or having bleeding diatheses.11
Acquisition of the Conventional MR Imaging Sequences
The MR imaging examinations were performed on 4 different scanners (two 1.5T and two 3T), by using a standard protocol that included axial T1WI, T2WI, FLAIR, DWI (with ADC maps), and CE-T1WI (CE-T1WI was available in 13 patients). The sequence parameters for FLAIR at 1.5T were the following: TR/TE/TI/NEX/echo-train length: 6500–9000 ms/105–110 ms/2000–2100 ms/1–2/15–23 and, at 3T, TR/TE/TI/NEX/echo-train length: 9000–11,000 ms/100–120 ms/2000–2100 ms/1–2/10–25 (5-mm section thickness). For DWI, the parameters at 1.5T were the following: TR/TE: 3300–4000 ms/71–120 ms and, at 3T, TR/TE: 2800–3000 ms/70–90 ms (5-mm section thickness); a gradient strength of b = 1000 s/mm2 was used for all DWI. For SWI, a routine sequence has been described previously, which combines magnitude and phase images to create the standard images reviewed.22 The parameters for volumetric acquisition of SWI at 1.5T were the following: TR/TE/NEX of 49/40 ms/1 with a voxel size of 1.2 × 0.8 × 2.0 mm (matrix size, 256 × 157 × 256) and, at 3T, TR/TE/NEX of 20 ms/27 ms/1 with a voxel size of 1.3 × 0.9 × 2.0 mm (matrix size, 256 × 134 × 256). Both the 1.5T and 3T acquisitions used a parallel imaging factor of 2, a flip angle of 15°, an FOV of 230 mm, and an acquisition time of approximately 3 minutes and were reconstructed at an axial thickness of 2.0 mm (20% gap). The resultant SWI images were reviewed by 2 methods: 1) by reviewing nonoverlapping axial 2.0-mm thickness images through the entire brain, and 2) by reviewing a minimum intensity projection of 20-mm thickness (2-mm overlap). The SWI images were reviewed in tandem with the other MR imaging sequences.
Image Interpretation
Two staff neuroradiologists with >7 years of experience in interpreting the MR imaging findings of PRES (A.M., C.L.T.) assessed the relevant findings on the available MR imaging studies by consensus in a joint review. If SWI was available on either a previous MR imaging before PRES (n = 7)or on a follow-up MR imaging (n = 17), those studies were reviewed in a similar fashion.
Classification of Severity on FLAIR Images
The 2 staff neuroradiologists determined the severity of PRES in all scans in consensus on the basis of a severity grading used in previous studies of PRES.2,3 According to that system, PRES was classified as mild, moderate, or severe:
Mild PRES.
Cortical or subcortical edema without IPH, herniation, or mass effect and with only minimal or no involvement of the cerebellum, brain stem, or basal ganglia.
Moderate PRES.
Confluent edema extending from the cortex to the deep white matter without extension to the ventricular margin or mild involvement of 2 of the following: cerebellum, brain stem, or basal ganglia. Mild mass effect but no herniation or midline shift, particularly if IPH was present, was also classified as moderate.
Severe PRES.
Confluent edema extending from the cortex to the ventricle, or edema or IPH causing midline shift or herniation. Alternatively, involvement of all 3 of the group, including the: cerebellum, brain stem, and basal ganglia, was considered severe.
Recording of Conventional MR Imaging Findings
The interpreters also determined “+” or “−” as to whether there was the presence of cytotoxic edema (reduced diffusion on DWI and ADC maps), sulcal hyperintensity on FLAIR (recorded as positive for SAH, if noted by the reviewers' consensus), SAH on SWI (sulcal hypointensity on SWI), and the presence of IPH (>5 mm size on SWI, if present). Additionally, the presence of contrast enhancement (leptomeningeal or parenchymal) was recorded if postcontrast T1WI was available (n = 15).
Recording of SWI Findings
The observers specifically recorded the following data with regard to the presence or absence of hemorrhage on SWI: 1) the presence or absence of any type of hemorrhage, 2) the type of hemorrhage (punctate <5 mm size = MH, >5 mm on SWI = IPH, or sulcal = SAH), and 3) the number of lesions if punctate foci of MH were detected. Notably, if the number of lesions was >20, it was recorded as “>20” because innumerable lesions could be nearly impossible to count. Lesions not occurring within regions of involvement by PRES were not tabulated, to remove the potential confounding factor of SWI-positive MHs being present in the asymptomatic population, whether related to underlying vasculopathies, cavernomas, or amyloid deposition in dementia-related disorders.23
Analysis of the Data
The incidence of hemorrhage was determined at presentation. For patients with a follow-up MR imaging or an MR imaging available before clinical presentation, the rate of persistence or development of these lesions was noted. A statistician used the Fisher exact test to evaluate significant associations between the following: the presence of MH on SWI- and DWI-positive lesions (“cytotoxic edema”), the presence of MH on SWI and SAH on FLAIR, the presence of MH on SWI and SAH on SWI, the presence of MH on SWI and IPH, and the presence of MH on SWI with the presence of contrast enhancement. A Wilcoxon signed rank test was performed to evaluate for an association between the presence of MHs on the initial MR imaging and MHs on the follow-up MR imaging in the 17 patients with follow-up SWI available. A Mann-Whitney U test was performed to evaluate for a significant difference between the number of MHs on SWI at 3T versus 1.5T. A Spearman test was also performed to determine the correlation between the number of MHs on SWI and the MR imaging severity. A P < .05 was used as the threshold for significance regarding all the tests described above.
Results
Patients
A total of 31 patients were identified with PRES clinically and on the basis of MR imaging findings. Of these, 16 had SWI at 3T, and 15, at 1.5T. Fig 1 briefly depicts the distribution of the SWI findings initially and at follow-up. Follow-up SWI was available in 17 patients; of these, 11 were performed at the same field strength.
Patients with PRES on SWI, with the frequency of MH and other subtypes of hemorrhage.
The average patient age was 37.9 ± 19.1 years. The most common clinical presentation of PRES was seizure (21/31 patients), followed by mental status change (4/31), headache (3/31), vision loss (2/31), and weakness (1/31). Regarding etiology, PRES was considered related to hypertension in 10 patients, toxicity from immunosuppressive medications post-transplantation in 8 (6/8 from cyclosporine, 2/8 from tacrolimus), sepsis in 5, eclampsia in 4, end-stage renal disease in 1, scleroderma in 1, lupus in 1, and thrombotic thrombocytopenic purpura in 1.
Conventional MR Imaging Findings and Follow-Up
At the initial presentation, MR imaging demonstrated “mild” PRES in 17/31 (54.8%) of patients, “moderate” PRES in 9/31 (29.0%), and “severe” PRES in (5/31) 16.1% (Figs 2–6 demonstrate examples of mild, moderate, and severe PRES). The follow-up MR images in the 17 patients with available imaging (with SWI images) were obtained within a range of 4–320 days (mean time to follow-up MR imaging, 63.8 ± 85.3 days). On follow-up MR imaging, the edema on FLAIR had improved or resolved in all patients; notably, 12 of these 17 cases had completely resolved on FLAIR. Of the 5 patients with some residual (albeit decreased) findings of PRES on the follow-up MR imaging, 2 patients had MR imaging performed at 5 days; 1, at 10 days; 1, at 12 days; and 1, at 30 days after initial presentation for MR imaging. According to our records, none of the patients died from PRES. DWI was positive for foci of reduced diffusivity in 7/31 patients (22.6%). Of the 15 patients with available postcontrast T1WI, 10/15 (66.7%) exhibited a pattern of leptomeningeal or cortical contrast enhancement.
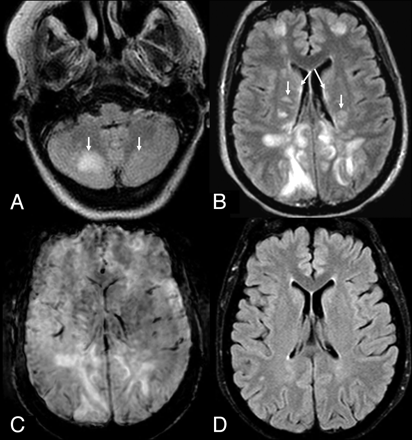
A 50-year-old woman with seizure and a history of hypertension who presented with “mild” PRES-related cortical and subcortical edema (dashed arrows) on a 3T FLAIR MR image (A), with a small underlying MH (arrow) on SWI (B). On a follow-up 3T FLAIR MR image (C), the PRES-related edema had mostly resolved, while the tiny MH persisted on SWI (D).
Presence of Hemorrhage
Findings positive for any type of hemorrhage were present in 20/31 patients (64.5%) on the initial/presenting MR imaging. Figure 1 depicts the frequency of each type of hemorrhage in patients with PRES, with MH in 18/31 (Fig 2), SAH in ≤4/31 (Figs 3 and 6), and IPH in 2/31 patients. MH was present in 11/17 patients (64.7%) who had an available follow-up SWI; SWI stayed positive for MH in each of those 11 patients on the follow-up MR imaging (Figs 2 and 5). However, in 1 of these 11 patients, the number of MHs decreased from 5 to 3 lesions. The remaining 56 MHs in the 11 patients with MHs on initial SWI did not change at follow-up. Notably, no new MHs developed in any of the 17 patients with an available follow-up MR imaging.
A 51-year-old hypertensive woman with unilateral moderate edema from PRES on 3T FLAIR images. This likely occurred unilaterally because the patient had a severe (>90%) left carotid bulb stenosis, which presumably prevented hyperperfusion of the left cerebral hemisphere. B, There is a small amount of SAH (arrows) on SWI. On a follow-up 3T MR imaging performed 70 days later, FLAIR image (C) demonstrated resolution of the PRES-related edema, while the SAH had also resolved on SWI (D).
A 48-year-old woman with severe extent of PRES on 1.5T FLAIR images (A and B), based on involvement of the cerebellum (arrows, A), basal ganglia (arrows, B), and brain stem (not shown) and because the cerebral edema extends from the ventricular margin to the cortex (B). Although the severity was denoted, SWI on that presenting MR image (C) did not demonstrate any MH. Follow-up FLAIR image (D) obtained on the same magnet 22 days later had nearly normal findings, and no MHs were noted on the follow-up SWI images either (not shown).
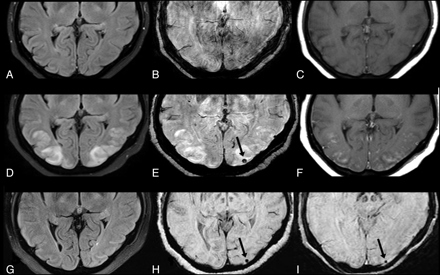
A 52-year-old patient with cyclosporine toxicity. Eleven days before the seizure, a pretransplant surveillance 3T MR imaging with FLAIR (A), SWI (B), and postcontrast T1WI (C) had normal findings. At presentation for seizure with mild PRES, 1.5T FLAIR (D) showed vasogenic edema, with a new punctate MH on SWI (E) and cortical and leptomeningeal contrast enhancement on postcontrast T1WI (F). After the episode of PRES clinically resolved, a 5-month follow-up MR image at 3T showed no edema on FLAIR (G), while the MH remained on SWI (H). The MH also persisted on a 1.5T SWI at 9 months (I).
Additionally, we further found that 7 of the 31 patients had SWI sequences performed at least 10 days before the onset of PRES (typically for pretransplant screening), none of whom had a history of PRES previously. Of these seven, 4 had previous negative findings on SWI but subsequently developed MH (Fig 5), while 2 of the 7 patients had MHs before PRES, which did not change in number or size after the patients developed PRES (Fig 6). None of the 7 had SAH or IPH before PRES.
A 3-year-old child with a seizure from tacrolimus toxicity post–heart transplantation. Thirty-five days before the seizure, a pretransplantation surveillance MR imaging at 1.5T had FLAIR images that appeared to have normal findings (not shown). A, At presentation with PRES, a 1.5T FLAIR image demonstrates edema of the caudate nuclei and frontal lobes (arrows) as well as bilateral parieto-occipital edema (not shown), considered moderate severity. There was dark SAH (dashed arrows, B) and multiple frontal, parietal, and occipital cortical/subcortical MHs (arrows) on SWI (B and C) at presentation, which totaled >20 MHs. On the follow-up 3T MR imaging 8 days later, the regions of vasogenic edema are nearly resolved on FLAIR images (not shown), while SWI demonstrates improved but persistent SAH (dashed arrows, D); the MHs also persist on SWI (D and E). F, Review of SWI from the 35-day pretransplantation 1.5T MR imaging reveals that each of the >20 MHs (arrows) were present before the onset of PRES.
Patients with Bleeding Diatheses or Anticoagulative Medications
Regarding patients with underlying bleeding diatheses, 6 patients had a low platelet count (<100 k), 4 of whom had MH on SWI (but no IPH or SAH); 1 of these patients had thrombotic thrombocytopenic purpura. Another 2 patients had an elevated INR >1.5 (1.7 and 1.9), 1 of whom had MH (but no IPH or SAH). Thus, 6 patients total were considered as having a predisposition to bleeding: 4 were positive for having MH on SWI and 1 of the 6 had SAH. Regarding medications for anticoagulation, 5 other patients were on such medications, 3 of whom were positive for MH on SWI; 1 had SAH noted only on FLAIR and another had SAH noted only on SWI.
Association of MH on SWI with SAH, DWI, Enhancement, and MR Imaging Severity
There was no significant association found between the presence of MH on SWI and the presence of the following: DWI-positivity (P = .667), SAH on FLAIR or SWI (both P > .999), IPH (P = .497), or contrast enhancement (P > .999). There was no significant difference between the presence of MH on SWI initially and the presence of MH at follow-up because all patients positive initially were likewise positive at follow-up (P = 1.00). The mean number of MH lesions on SWI based on the initial MR imaging at 3T was 3.44 ± 5.31, and at 1.5T, it was 2.60 ± 4.92. There was no significant difference between the number of lesions detected at 3T versus 1.5T (P = .564). No correlation existed between the number of MHs on SWI and the MR imaging severity (r = 0.077, P = .68).
Discussion
SWI is a novel sequence, which has shown promise in a variety of clinical settings, including the depiction of MHs, SAH, deoxygenated blood, calcium, and even metallic deposits.12⇓–14,22,24⇓⇓–27 It was originally developed for MR venography to optimize small-vessel visualization but soon gained more widespread use in detecting MHs, such as in hemorrhagic shear (diffuse axonal) injury.12,13,26 Although a relatively new method, it has so far notably provided additional insightful information not readily available on conventional T2*GRE because it has demonstrated an ability to detect MHs that is superior to both T2*GRE and nonenhanced CT.12⇓–14 Thus, preliminary evidence has demonstrated that the use of SWI to depict MH may improve the prognostic accuracy in important clinical scenarios, such as predicting long-term outcomes in children with either accidental or nonaccidental trauma.12,18 Other potential clinical uses of SWI have recently included grading of primary brain tumors, detecting cerebral amyloid-related MH, and detecting or characterizing underlying vascular malformations, to name a few.22,27⇓⇓–30 Hence, there could also be potential application in depicting MH in PRES, in which prior studies have described hemorrhage in 15%–17% of affected patients.2,11 In this study, we set out to assess whether there was increased detection of hemorrhage by SWI in the setting of PRES, and we found a much higher frequency of hemorrhage, typically MH, than previously described. However, we also found that this high incidence of MH in SWI does not appear to correlate with the MR imaging severity/extent of edema, the presence of DWI-positive findings, or the presence of enhancement on T1WI. Additionally, most MH lesions appear to persist on long-term follow-up. The presence of hemorrhage in PRES is important to note because patients with PRES-related IPH may potentially have poor outcomes.31 Thus, although the clinical significance of such MHs on SWI in PRES on the initial MR imaging examination remains undetermined, our preliminary evidence would suggest that the number of MHs does not appear to relate to the clinical severity.
We also found a rate of SAH and IPH similar to that in previous studies, though SAH was present in this study on SWI in 3 patients and on FLAIR in 4 patients (2 of whom had positive findings on both sequences). It has not yet been determined whether FLAIR or SWI or even CT is more sensitive for SAH, though early evidence would suggest that SWI is slightly more sensitive than CT for traumatic SAH, particularly if intraventricular.24 We admit that our classification of sulcal hyperintensity on FLAIR as SAH was based on studies before the routine use of SWI; thus, there is no direct evidence that such sulcal hyperintensity on FLAIR in patients with PRES truly represents SAH.2,11
Regarding the etiology of hemorrhage in PRES, a review of all of the potential pathogenetic mechanisms is beyond the scope of this study, but a short review of the proposed mechanisms resulting in the typical vasogenic (and occasionally cytotoxic) edema in PRES may reveal the cause of MH, SAH, and the uncommon IPH. In short, an ongoing controversy exists regarding the etiology of PRES, in which many mechanisms have been proposed, but the 2 prevailing theories involve the original “hypertension and hyperperfusion” theory versus the revisited concept of “endothelial dysfunction” (which may entail hypoperfusion).32 The former and previously more popular mechanism involved hyperperfusion with failed autoregulation, in which elevated “breakthrough” pressure overwhelming the microvascular endothelium would lead to vasogenic edema and potentially hemorrhage; however, this theory has been challenged because more recent evidence using perfusion examinations, such as MR perfusion and SPECT, suggests that hyperfusion may not be present, because hypoperfusion has often been noted.33⇓⇓⇓⇓⇓–39 Also, the lack of elevated blood pressure in many patients, such as in the immune-suppressed population or those with sepsis, indicates that this theory is not all-inclusive and that some other underlying factor must be present.2,40 Thus, recent studies of immunosuppressant medications suggest that the mechanism of PRES relates to endothelial cell damage or dysfunction, leading to blood-brain barrier impairment, with resultant cortical and subcortical vasogenic edema; such dysfunction could explain the high frequency of contrast enhancement that typically reverses.2,41⇓–43 Endothelial dysfunction could also explain not only the regions of reversible cortical/subcortical edema and the high prevalence of enhancement but may also explain the 3 types of hemorrhage that have been reported in PRES: 1) punctate MHs, 2) sulcal SAH, and 3) lobar IPH.2,11 Of those subtypes, punctate MHs are expected to be the most difficult to discern and thus would necessitate the use of SWI for detection. Notably, a previous study by Hefzy et al11 reported an incidence of hemorrhage in 15.2% of patients with PRES, with MHs in 52% (n = 12/23) of patients who had hemorrhage, as detected on T2*GRE. McKinney et al2 noted a similar frequency of hemorrhage in 17.3% (n = 13/76) of patients with PRES, but they did not report the frequency of MHs. The current study found a much higher frequency of MHs in PRES, very likely due to the improved accuracy of SWI.
This study is subject to a number of potential limitations, particularly those related being retrospective. First, there could be problems with either cases of PRES that were not identified or patients mistakenly diagnosed with PRES, which could skew the data. For this reason, we included only patients with both a clinical and imaging course consistent with PRES. Second, we included only patients with the typical cortical/subcortical vasogenic edema, thus potentially excluding more severe cases with brain stem-only or basal ganglia-only involvement, because it could be difficult to distinguish MHs located centrally from normal basal ganglia iron or calcium. These strict criteria for inclusion along with the retrospective nature of this study (potentially excluding severely affected patients radiologically and clinically) made it difficult to correlate the severity of imaging findings with the clinical severity. Third, SWI dark foci may be present in cavernomas, amyloid deposits, and vasculopathic disorders; this presence could also skew the data if there was a high incidence of such diseases in our PRES population. Fourth, there is heterogeneity of the availability of both pre- and post-PRES MR imaging, in which a baseline MR imaging with SWI was present in only a minority of the patients and there was a lack of follow-up imaging in a significant number of the patients. Fifth, the use of 2 different field strengths may be considered a technical limitation because SWI results can differ with field strength.15,16 However, we did not visually notice a difference between MHs with different field strengths. We also did not note any MHs “disappearing” between field strengths in the same patient, and no significant difference was found between the number of MH lesions detected on SWI at 3T versus 1.5T (eg, Figs 5 and 6). Sixth, we acknowledge that a consensus review by 2 staff neuroradiologists does not account for potential variability in image interpretation in PRES, in particular regarding subtle findings such as SAH. However, we used a method of consensus review by 2 experienced staff neuroradiologists that is similar to that in previous larger retrospective studies of hemorrhagic PRES.2,11
Finally, another limitation could be expectation bias because this study was formulated on the basis of clinical observations and the possible expectation of an increased detection rate of MH. However, we think that the joint review by 2 neuroradiologists experienced in interpreting MR imaging findings of PRES should compensate for this potential limitation.
Conclusions
Hemorrhage, in particular MH, occurs in PRES at a rate higher than previously described, on the basis of SWI. It appears that most MHs persist chronically after the PRES-related vasogenic edema resolves. Our preliminary evidence suggests that some MHs are already present before PRES, possibly related to an underlying vasculopathy, but new lesions do develop in many patients with PRES and persist for a long time thereafter. Additionally, our preliminary evidence indicates that the presence of MH on SWI does not correlate with any other particular imaging findings or the resolution of symptoms, so the clinical significance, if any, of these MHs in the setting of PRES remains unknown and should be studied further.
Footnotes
Disclosures: Charles Truwit—UNRELATED: Travel/Accommodations/Meeting Expenses: Vital Images, Phillips Healthcare, Comments: advisory board meetings.
References
- Received July 5, 2011.
- Accepted after revision August 15, 2011.
- © 2012 by American Journal of Neuroradiology