Abstract
SUMMARY: Ataxia-telangiectasia, an autosomal recessive disorder caused by defect of the ataxia-telangiectasia mutated gene, is characterized by progressive neurologic impairment with cerebellar atrophy, ocular and cutaneous telangiectasia, immunodeficiency, heightened sensitivity to ionizing radiation and susceptibility to developing lymphoreticular malignancy. Supratentorial brain abnormalities have been reported only rarely. In this study, brain MRI was performed in 10 adults with ataxia-telangiectasia having stable neurologic impairment. Intracerebral telangiectasia with multiple punctate hemosiderin deposits were identified in 60% of subjects. These lesions were apparently asymptomatic. They are similar in appearance to radiation-induced telangiectasia and to cryptogenic vascular malformations. Also noted, in the 2 oldest subjects, was extensive white matter T2 hyperintensity, and in 1 of these a space-occupying fluid collection consistent with transudative capillary leak and edema as evidenced by reduced levels of metabolites on MR spectroscopic imaging. Asymptomatic supratentorial vascular abnormalities appear to be common in adults with ataxia-telangiectasia.
ABBREVIATIONS:
- A-T
- ataxia-telangiectasia
- AFP
- alpha-fetoprotein
- ATM
- ataxia telangiectasia mutated
- MRSI
- MR spectroscopic imaging
Ataxia-telangiectasia (A-T) is an autosomal recessive neurodegenerative disorder associated with a single defective gene localized to chromosome 11 (11q22–23)1 that is estimated to affect 1 in 40,000–300,000 people.2,3 The causative gene, termed ataxia telangiectasia mutated (ATM), is constitutively expressed in all eukaryotic cells and encodes a serine-threonine kinase key to a number of important cellular responses, including the DNA damage response and associated cell-cycle checkpoint regulation.4 The range of clinical features manifested by people with A-T is similarly broad and dramatic, including telangiectatic vessels on the bulbar conjunctivae and skin, humeral and cell-mediated immunodeficiency, and an array of cerebellar and other neurologic impairments.5 Affected persons also manifest both heightened sensitivity to ionizing radiation and enhanced susceptibility to malignant disease, chiefly lymphoreticular in nature.6⇓–8
MR imaging is the favored technique of neuroimaging for patients suspected to have A-T because of its superior tissue contrast, as well as the absence of ionizing radiation. Cerebellar atrophy is the most consistent finding, though it is usually not present in the preschool years when most patients first seek neurologic consultation.9 The supratentorial brain is typically normal on MR imaging.10,11 A few cases have been reported of patients with A-T, mostly adults surviving into their second and third decades of life, in whom cerebral white matter abnormalities of 2 types have been described. One pattern consists of multiple T1 and T2 hypointense foci suggestive of hemosiderin, thought to be related to thrombosis and vascular leaks from multiple capillary telangiectasias.12 The other pattern, described in a single child and in a few adult patients,11,13⇓–15 is T2/FLAIR hyperintensity on long TR sequences that simulates leukodystrophy. The purpose of this study was to analyze the MR imaging features of the supratentorial brain in a small series of young adults with A-T, all otherwise healthy and neurologically stable for years, though they were manifesting substantial persistent neurologic deficits.
Case Series
This study was approved by the local institutional review board (NA_00014314 and NA_00012201). Ten adult participants with A-T (4 men, 6 women; mean age, 23.4 ± 4.5 years; age range, 19–34 years; Table) constitute the case series here reviewed. Eight of these patients were enrolled in a research study16 to collect CSF, and they underwent MR imaging scans as a safety measure before lumbar puncture. The ninth participant was neurologically stable but underwent clinical MR imaging to evaluate chronic headaches. The final participant underwent MR imaging for clinical concerns but had experienced no new symptoms or signs in several years. The research brain MR imaging studies were performed at 3T and consisted of T1, T2, and SWI. For the 2 clinical studies, in addition to conventional sequences, proton MR spectroscopic imaging (MRSI; TR, 1500 ms; TE, 280 ms) was acquired at 1.5T.
Characteristics of adults with ataxia-telangiectasia
All participants were followed in the Ataxia-Telangiectasia Clinical Center at our institution and underwent MR imaging between 2005 and 2008. At the time of MR imaging evaluation, all participants had stable neurologic impairments of variable severity, including ataxia, dystonia, various forms of tremor, dysarthria, oculomotor abnormalities, distal diminished sensibility to light touch and position, and decreased or absent distal tendon reflexes. All used a wheelchair for mobility. The diagnosis of A-T was by fulfillment of established clinical criteria,5,9 including these and other characteristic clinical features, and laboratory studies of elevated alpha-fetoprotein (AFP); increased lymphocyte chromosomal breaks after in vitro radiation exposure; and, in some cases, demonstration of absent ATM protein on Western blot analysis or demonstration of predicted protein-null homozygous mutations of ATM (Table).
On MR imaging, all participants had manifest cerebellar atrophy, whereas the supratentorial brain showed no sign of volume loss. In 6 participants, SWI showed few to innumerable punctate signal voids, suggesting hemosiderin deposits, scattered throughout the cerebral white matter. Four of these 6 were in the research-acquired group, and in these 4 participants, the punctate lesions were inconspicuous on other pulse sequences, including T2-weighted FSE images. In the 2 participants (participants 9 and 10) scanned for clinical concerns (but, like the research-acquired group, absent of any recent change in neurologic function) additional findings of white matter T2 hyperintensity were present, surrounding and highlighting some of these lesions. No signal abnormalities were evident in the cerebellum or brain stem.
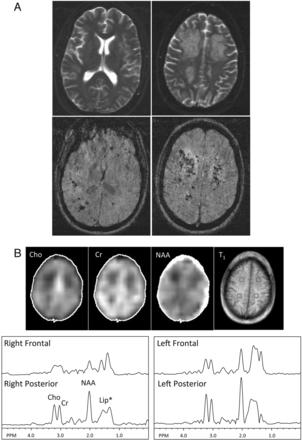
In participant 9 (Fig 1), a 34-year-old man who was evaluated for chronic headache, innumerable punctate lesions were best seen on SWI. Several clusters of these lesions were surrounded by T2/FLAIR hyperintensity in the white matter and were associated with mild mass effect (Fig 1A). In these regions of white matter hyperintense T2 signal abnormality—in contrast to the surrounding normal-appearing white matter—1H-MRSI showed a diminution of all examined metabolites, suggesting reduced cellularity with edema, or perhaps gliosis (Fig 1B). No increase of choline was evident, arguing against an active demyelinating process. No abnormal elevation of lactate was found in any of the brain regions or in the CSF.
MR imaging and MRSI in participant 9, a 34-year-old man with A-T who had experienced chronic headaches. A, Top: T2-weighted images show multifocal confluent hyperintensity in the frontal and parietal white matter bilaterally. A few punctate hypointense foci suggestive of hemosiderin deposits can be identified in the left frontal white matter. Bottom: SWI shows innumerable foci of susceptibility consistent with hemosiderin deposits scattered throughout the bilateral cerebral white matter, some clustered within the confluent T2 hyperintensity. B, MRSI metabolite maps (Cho = choline, Cr = creatine, NAA = N-acetylaspartate) and corresponding T1-weighted MR imaging showing voxel locations for selected spectra in the left and right frontal and posterior white matter. Lower levels of Cho, Cr, and NAA are seen, particularly in the right frontal region corresponding to T2 hyperintense white matter, compared with the spectra obtained from the posterior centrum semiovale where more normal-appearing white matter was found. Note the broad peak (approximately 1.5 ppm) upfield from NAA in all brain regions is related to scalp lipid contamination that results from head motion during the scan.
In participant 10 (Fig 2), a 28-year-old man who underwent MR imaging simply because of concern for his advancing age, there was an ovoid T2 hyperintense and T1 hypointense space-occupying lesion lined by hemosiderin deposits and surrounded by vasogenic edema (Fig 2A). Some of the T2 dark lesions showed contrast enhancement, suggesting telangiectatic vessels. Within the surrounding white matter T2 hyperintensity, MRSI (Fig 2B) again showed the decrease of all metabolites in a pattern similar to that apparent in participant 9. The right frontal lesion was virtually devoid of any metabolites, consistent with a fluid collection.
A 28-year-old man with no recent changes in neurologic function underwent scanning because of concern for advancing age. A, A T1 hypointense, T2 hyperintense, mild rim-enhancing lesion in the right parietal white matter, with surrounding T2 hyperintensity suggestive of vasogenic edema. The contrast enhancement corresponds to punctate T2 hypointense hemosiderin deposits that outline the ovoid lesion, in addition to a few isolated foci, suggestive of telangiectasia. B, MRSI shows complete signal void in the right parietal ovoid lesion (ROI 3), compatible with a fluid collection. Within the surrounding white matter T2 hyperintensity, all metabolites are decreased (ROI 2), compared with normal-appearing white matter regions (ROIs 1 and 4). Residual lipid signals upfield from NAA are the result of head motion.
Discussion
This case series identifies a spectrum of supratentorial white matter findings in adults with A-T. Combined with the experience of a few reported individual cases,12,17 it is apparent that patients with A-T frequently manifest scattered small white matter hypointensities that most likely represent tiny hemosiderin deposits related to telangiectatic vessels. These lesions were most conspicuous on SWI. The presence of these asymptomatic lesions in 6 of 10 neurologically stable patients > 19 years old contrasts with their absence in a younger reported cohort18 of 8 patients with A-T (mean age, 13 years; age range, 9–19 years), as well as our general experience at the Johns Hopkins Ataxia Telangiectasia Clinical Center reviewing several hundred initial diagnostic MR imaging scans. Although age-related differences in sensitivity or other technical differences are possible confounders, the most plausible explanation is that these are acquired lesions, passing over a threshold of MR imaging detectability in early adult years.
Disabling mutation of the ATM gene responsible for A-T has pleotrophic cellular effects.4,19,20 Among the most prominent of these is disruption of the cell-cycle arrest and other cellular responses to DNA damage. Identification of the specific impaired ATM-kinase pathway responsible for formation of deep white matter telangiectasia is unknowable, though this is an appropriate target for research. The ocular and cutaneous telangiectasias that are part of the defining feature of A-T appear in light-exposed superficial regions. A similar vascular pathologic pattern in ATM-deficient mice21 that arises in the retina with diminished retinal vasculature attenuation is associated with increased vascular endothelial growth factor expression (implicated in angiogenesis), decreased tight junction protein occludin expression, and perturbed astrocytic interaction with endothelial cells, as well as increased vascular permeability with deposits of hemosiderin; however, this model differs from the deep brain lesions demonstrated in this case series by its relationship to light exposure.22 Another plausible explanation is offered by growing evidence that ATM mediates vascular endothelial cell senescence.23,24
The imaging findings reported here in A-T are reminiscent of those seen in radiation-induced vascular malformations and white matter injuries. Vascular telangiectasias are reported more frequently in patients after CNS irradiation.25⇓–27 Parenchymal radiation-associated MR imaging changes typically manifest as progressive, mild to moderate T2 prolongation in the periventricular white matter,25 which may be the result of vascular abnormalities leading to parenchymal ischemia and white matter degeneration. It is not clear, however, whether similar mechanisms cause the white matter changes seen in older patients with A-T.
An unusual finding, identified in our 2 oldest participants (ages 34 and 28 years), was that of white matter T2/FLAIR hyperintensities. Similar hyperintensities have been the topic of individual case reports,11,14,15 although we believe that these differ from the single case report of a child with diffuse white matter signal simulating leukodystrophy,13 a pattern we have not otherwise seen. Although MR spectroscopy and MRSI have been previously reported in A-T,17,18 abnormalities (eg, reduced NAA) were found only in the atrophic cerebellum, and MR spectroscopy abnormality has not previously been reported in the supratentorial compartment. In one of the reported cases,14 these white matter signal changes were thought to represent demyelination, but the associated low level of all metabolites found in our MRSI investigation of similar T2 appearance lesions suggests that these white matter signal abnormalities represent reduced cellularity rather than active demyelination or ischemia. The peculiar appearance of a discrete fluid-filled cavity surrounded by the hemosiderin lesions in participant 10 of our series can be best explained by a transudative or exudative process related to leaky capillary telangiectasia.
A spectrum of supratentorial MR imaging abnormalities seem to be common in adults with A-T. These are asymptomatic, or possibly their associated signs and symptoms are lost amid the many otherwise stable neurologic impairments that such patients endure. Six of 10 participants in our current study manifested deep cerebral telangiectatic vessels best visualized on the SWI sequence, whereas the 2 oldest participants also manifested parenchymal lesions and white matter signal abnormalities. The vascular lesions have many similarities to those seen in patients after therapeutic radiation. The pathogenesis of these lesions is unknown, but candidates include impaired DNA damage response, oxidative damage, enhanced senescence, or any combination of these 3 changes. The prognosis of these MR imaging changes is unclear and will require longitudinal studies.
Footnotes
Disclosures: Howard Lederman—RELATED: Grant: A-T Children's Project*; Support for Travel to Meetings for the Study or Other Purposes: A-T Children's Project.* *Money paid to institution.
This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by grant number UL1 TR 000424-06 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS, or NIH. Additional support was provided by NIH grant P41EB015909 and the Ataxia Telangiectasia Children's Project, Boca Raton, Florida.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received May 31, 2011.
- Accepted after revision March 13, 2013.
- © 2014 by American Journal of Neuroradiology