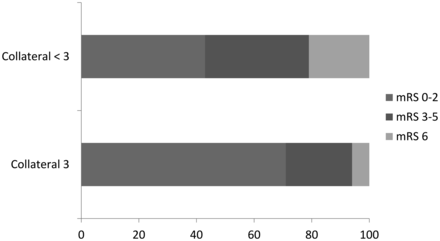Abstract
BACKGROUND AND PURPOSE: Although intra-arterial therapy for acute ischemic stroke is associated with superior recanalization rates, improved clinical outcomes are inconsistently observed following successful recanalization. There is emerging concern that unfavorable arterial collateralization, though unproven, predetermines poor outcome. We hypothesized that poor leptomeningeal collateralization, assessed by preprocedural CTA, is associated with poor outcome in patients with acute ischemic stroke undergoing intra-arterial therapy.
MATERIALS AND METHODS: We retrospectively analyzed patients with acute ischemic stroke with intracranial ICA and/or MCA occlusions who received intra-arterial therapy. The collaterals were graded on CTA. Univariate and multivariate analyses were used to investigate the association between the dichotomized leptomeningeal collateral score and functional outcomes at 3-months mRS ≤2, mortality, and intracranial hemorrhages.
RESULTS: Eighty-seven patients were included. The median age was 66 years (interquartile range, 54–76 years) and the median NIHSS score at admission was 18 (interquartile range, 14–20). The leptomeningeal collateral score 3 was found to have significant association with the good functional outcome at 3 months: OR = 3.13; 95% CI, 1.25–7.825; P = .016. This association remained significant when adjusted for the use of IV tissue plasminogen activator: alone, OR = 2.998; 95% CI, 1.154–7.786; P = .024; and for IV tissue plasminogen activator and other confounders (age, baseline NIHSS score, and Thrombolysis in Cerebral Infarction grades), OR = 2.985; 95% CI, 1.027–8.673; P = .045.
CONCLUSIONS: We found that poor arterial collateralization, defined as a collateral score of <3, was associated with poor outcome, after adjustment for recanalization success. We recommend that future studies include collateral scores as one of the predictors of functional outcome.
ABBREVIATIONS:
- IAT
- intra-arterial therapy
- IQR
- interquartile range
Intravenous tissue plasminogen activator is the only proved reperfusion therapy for acute ischemic stroke. However, a narrow therapeutic time window (<4.5 hours) limits its use because the clinical effectiveness is critically time-dependent.1⇓–3 In addition, recanalization rates with IV-tPA are low in the setting of large-artery occlusion, (eg, ICA occlusion <10%).4⇓–6 Intra-arterial therapy (IAT) has higher recanalization rates than intravenous thrombolysis, but this result has not been matched by concordant improvement in clinical outcomes.7⇓–9 Two recent randomized trials comparing IAT with IV-tPA, the Interventional Management of Stroke III trial and the Local versus Systemic Thrombolysis for Acute Ischemic Stroke trial, did not demonstrate superiority.10,11
Inadequate arterial collateralization is a possible mechanism to explain the mismatch between recanalization success and clinical outcome, apart from the presence of an already infarcted ischemic core and an incomplete microcirculatory reperfusion after focal cerebral ischemia.12,13 A favorable arterial collateralization as determined by a robust leptomeningeal anastomoses profile may enhance recanalization, improve downstream reperfusion, reduce the extent of infarct core and ischemic lesion growth, decrease hemorrhagic transformation, and improve outcome postrevascularization.14⇓–16
The leptomeningeal collateral scoring system based on CTA correlates with clinical outcome.17⇓⇓⇓–21 However, its role in IAT is unclear. We hypothesized that a poor leptomeningeal CTA score predicts clinical futility in patients undergoing IAT independent of recanalization status.
Materials and Methods
Study Design and Patient Cohort
This was a single-center retrospective review of 104 patients with acute ischemic stroke with ICA and/or MCA occlusion who received IAT at the Royal Melbourne Hospital from January 2008 to March 2013. The local research and ethics committee granted the study approval.
Clinical parameters, identified through computerized data bases, were prospectively collected. The following parameters were recorded in a specific data bank: 1) demographics (age, sex); 2) medical history and risk factors such as hypertension (previous clinical diagnosis or regular treatment with antihypertensive medication), diabetes mellitus (previous diagnosis or current treatment with insulin or oral hypoglycemic medication), hypercholesterolemia (previous diagnosis or current treatment with lipid-lowering medication), atrial fibrillation (previous diagnosis or evident on admission), coronary artery disease, history of previous stroke or TIA; 3) “time from onset of symptoms to CTA” (defined from the time of symptom onset or from the time when the patient was last seen neurologically well, to the time of CTA), “time from onset of symptoms to recanalization” (defined from the time of symptom onset, or from the time when the patient was last seen neurologically well, to the time of intervention with any form of IAT), administration of IV-tPA before endovascular therapy, administration of intra-arterial tPA; and 4) clinical outcome variables: 3-month mRS score, intracranial hemorrhage, and 1-month mortality. The baseline severity of neurologic deficits was graded on admission according to the NIHSS.
CTA and DSA Analysis
CTAs of all patients were reviewed by 3 neurointerventionists (B.Y., P.M., and R.D.), with consensus opinion reached on collateral supply. The reviewers were blinded to all clinical information during the consensus collateral grading. The CTA source images (20-mm, axial) were edited with MPR technique for assessment of leptomeningeal collaterals based on distribution of vessels at the Sylvian fissure and the leptomeningeal convexity. The MPR images were assessed with an MIP technique at 32 mm to evaluate the leptomeningeal collaterals. Collateral statuses were divided into 4 categories (Fig 1): score 0 = absence of contrast reaching the cortical surface of the affected hemisphere; score 1 = contrast reaching the cortical surface but not the Sylvian fissure; score 2 = contrast reaching the Sylvian fissure but opacifying <50% of hemisphere; score 3 = contrast reaching the Sylvian fissure and opacifying >50% of the hemisphere.
Leptomeningeal collateral CTA scores; A, score = 0; B, score = 1; C, score = 2; D, score = 3.
The Thrombolysis in Cerebral Infarction system was used to grade recanalization success.22 TICI grade 0 is no perfusion and no antegrade flow beyond the point of occlusion. TICI grade 1 is penetration with minimal perfusion. The contrast material passes beyond the area of obstruction but fails to opacify the entire cerebral bed distal to the obstruction for the duration of the angiographic run. TICI grade 2 is partial perfusion. The contrast material passes beyond the obstruction and opacifies the arterial bed distal to the obstruction. However, the rate of entry of contrast into the vessel distal to the obstruction or its rate of clearance from the distal bed or both are perceptibly slower than its entry into and/or clearance from comparable areas not perfused by the previously occluded vessel (eg, the opposite cerebral artery or the arterial bed proximal to the obstruction). In TICI grade 2a, only partial filling (less than two-thirds) of the entire vascular territory is visualized; in TICI grade 2b, complete filling of all of the expected vascular territory is visualized, but the filling is slower than normal. TICI grade 3 is complete perfusion; antegrade flow into the bed distal to the obstruction occurs as promptly as that into the obstruction and clearance.
Statistics
Statistical analysis was performed by using the STATA, Version 12 IC statistics package (StataCorp, College Station, Texas). We included the following baseline clinical variables: age, sex, comorbidities (history of hypertension, diabetes, atrial fibrillation, coronary artery disease, TIA, or stroke), baseline NIHSS score, IV-tPA, intra-arterial tissue plasminogen activator, onset time to CTA, and onset time to recanalization. Continuous variables were reported as median ± interquartile range (IQR). Categoric variables were reported as proportions. P values were reported by using the Fisher exact test for categoric variables and the Wilcoxon-Mann-Whitney rank sum test for continuous variables (age, NIHSS score, and time-to-event data). Effect sizes were reported as odds ratios for categoric variables and Hodges-Lehman median differences for continuous variables (age, NIHSS score, and time-to-event data). Receiver operating characteristic analysis curves were used to determine the collateral score threshold with functional outcome (3-month mRS score). Univariate and multivariate analyses were used to investigate the association between the dichotomized leptomeningeal collateral score with functional outcomes, mortality, and intracranial hemorrhages. Given the strong clinical association with clinical outcomes, we planned a priori to include the following independent variables in the model: age, baseline NIHSS score, TICI grade, and IV-tPA. A 2-sided P value <.05 was considered significant.
Results
Of the initial 104 patients with acute ischemic stroke with ICA and/or MCA occlusion who underwent IAT, CTA was not available for 14 patients before IAT. Three patients had poor image quality on CTA, and these patients were excluded. Eighty-seven patients with both adequate CTA and angiographic data with clinical outcome (mRS) at 90 days available were entered into the leptomeningeal collateral assessment. The baseline characteristics did not differ in a statistically significant manner between the patients with and without CTA (Table 1). In the CTA group, the median age was 66 years (IQR, 54–76 years). Fifty (57%) patients were men. The median NIHSS score at admission was 18 (IQR, 14–20). Forty (46%) subjects were treated with IV-tPA; 13 subjects (15%) received intra-arterial tPA. Sixty-five patients (75%) were treated with the Solitaire device (Covidien, Irvine, California), 10 (12%) patients were treated with the Merci retriever (Concentric Medical, Mountain View, California), and 3 (3%) were treated with the Penumbra System (Penumbra, Alameda, California).
Baseline characteristics of the patient cohort with and without CTA
Leptomeningeal Collaterals
According to the leptomeningeal score, 34 subjects (39%) were graded as three, 30 subjects (35%) were graded as two, 12 subjects (14%) were graded as 1, and 11 subjects (13%) were graded as zero. Receiver operating characteristic analysis demonstrated the suitability of the collateral score ≥3 for determining good functional outcome of mRS ≤2 with an area under the curve of 0.6513 (sensitivity, 51.1%; specificity, 73.8%). The leptomeningeal score was dichotomized into good (score = 3) and poor (score = 0–2) on the basis of the receiver operating characteristic analysis. A higher percentage of IV-tPA was administered for a collateral score of 3 than for a collateral score of 0–2, (P = .008). The remainder of the baseline clinical variables did not differ in a statistically significant way between the 2 groups (Table 2).
Comparison of patients for the dichotomized leptomeningeal collateral score
Outcome
Three-month outcome was favorable (mRS 0–2) in 47 subjects (54%), and the remaining 40 subjects (46%) had mRS scores of 3–6 (Fig 2). Patients with a good collateral score of 3 had higher odds of good functional outcome than patients with a collateral score of 0–2 (OR = 3.130; 95% CI, 1.252–7.825; P = .016). This finding remained significant after adjustment for administration of IV-tPA (OR = 2.998; 95% CI, 1.154–7.786; P = .024). In the multivariate analysis, patients with a collateral score of 3 had significantly higher odds of good outcome at 3-month follow-up after adjustment for age, baseline NIHSS score, IV-tPA, and TICI grades (adjusted OR = 2.985; 95% CI, 1.027–8.673; P = .045) (Table 3).
Distribution of mRS scores according to the leptomeningeal collateral scores.
Univariate analysis of associations for outcomes for the dichotomized leptomeningeal score
Survival
Of the 13 deaths at 1 month, patients with poor collateral scores of 0–2 exhibited a trend toward a higher death rate than those with a collateral score of 3 (11 versus 2) (OR = 0.239; 95% CI, 0.049–1.153; P = .070), though no statistically significant differences were found.
Intracranial Hemorrhage
Although patients with a good collateral score of 3 had fewer episodes of intracranial hemorrhage than patients with a poor collateral score of 0–2 (4 versus 13), no statistically significant difference was detected (OR = 0.41; 95% CI, 0.122–1.385; P = .174).
Discussion
We found that a favorable pattern of leptomeningeal collaterals, as measured by CTA on admission, was associated with improved functional outcomes at 3 months in a cohort of patients with acute ischemic stroke with ICA or proximal MCA occlusion. In a multivariate model, a good leptomeningeal collateral score remained independently associated with good clinical outcome after adjustment for age, baseline NIHSS score, IV-tPA, and recanalization status.
Various studies have used CTA to score collaterals status14,17,19,20,23 with either CTA source images or by using MIP. Tan et al17 demonstrated that CTA-MIP was the best technique to quantify the degree of collateral circulation. In their retrospective analysis, they reported a good correlation between the CTA-based collateral score and the final infarct volume within 6 months.17 In another study, Miteff et al14 assessed retrograde filling of the MCA by 3 categories of collateral scoring and concluded that a good collateral status was one of the significant univariate predictors of favorable outcome.14 Menon et al21 included 138 patients (MCA-M1 and/or intracranial occlusion) in a retrospective single-center study and demonstrated good regional leptomeningeal anastomoses scores in 37.6% of patients, which correlated strongly with the size of the infarct core at baseline and were a strong independent predictor of final infarct and clinical outcome.
In a recent study, Souza et al23 found that CTA collaterals correlate with admission diffusion-weighted imaging infarct size and that a malignant collateral profile is highly specific for large admission DWI lesion size and poor functional outcome. In another study, Angermaier et al24 demonstrated that the CTA collateral grade was an independent predictor of final infarct volume in patients with stroke treated with endovascular therapy. On the other hand, Rosenthal et al18 reported that CTA collaterals had a positive impact on the outcomes of patients who did not achieve complete recanalization and had no impact in patients who were completely recanalized. Tan et al25 reported that good CTA collaterals correlated with improved outcomes in uni- but not in multivariate analyses. Our study reveals that a good CTA collateral score had a positive impact on the outcomes of patients in both uni- and multivariate analyses independent of recanalization status.
The system of leptomeningeal anastomoses is anastomotic connections between distal branches of the cerebral arteries on the surface of the brain that permit blood flow from the territory of an unobstructed artery into the territory of an occluded artery and constitute the secondary network of cerebral collateral circulation apart from the circle of Willis.26 Although the criterion standard for the assessment of leptomeningeal anastomoses is conventional DSA, the extent of leptomeningeal anastomoses formation seen on conventional DSA and with clinical outcome correlates well with collateral blood flow as assessed by CTA.15,27,28 CTA is quicker, simpler, and noninvasive and uses IV-administered contrast to visualize the extent of leptomeningeal anastomoses with intra- and extracranial vasculature.19,21
There is currently no consensus on what the presence or absence of collateral circulation means with respect to treatment choices for patients with acute ischemic stroke. Some authors believe a minority of patients with robust leptomeningeal collateral circulation will have minimal damage and experience excellent recovery without recanalization.29 On the other hand, some neurointerventionalists may want to try more aggressive IAT because there may be salvageable tissue in patients with good collateral vessel formation. Given the risks inherent in the therapeutic procedure and the clinical risk of futile recanalization,30 practitioners need a way to consistently select patients whom IAT is likely to benefit rather than harm. Given the significance of leptomeningeal collaterals, they should be taken into account with other validated scoring systems, namely the Houston Intra-Arterial Therapy 30 and the Totaled Heath Risks in Vascular Events score,31 in patient selection for IAT.
Our study has some limitations. First, selection bias is unavoidable in a retrospective single-center study. We sought to minimize reading bias by blinding the observers to clinical data and consensus achieved for the collateral grading. The CTA collateral scoring system was modified from Tan et al.17 Although our collateral grading derived from a consensus by 3 experienced neurointerventionists, the utility and value of the score requires further validation in a larger study before collateral scores are used as a primary means of selecting patients for revascularization therapy. Potential biases may have influenced the selection of cases for IAT on the basis of the CTA and clinical data.
Conclusions
Evaluation of the leptomeningeal collateral blood supply before IAT for anterior circulation intracranial arterial occlusion stroke (ICA and/or MCA) with a collateral score based on CTA-MIP reconstructions is independently associated with functional outcome at 3 months. We believe that leptomeningeal collateral assessment should be included in future trials investigating the clinical efficacy of IAT.
References
- Received July 25, 2013.
- Accepted after revision August 26, 2013.
- © 2014 by American Journal of Neuroradiology