Abstract
BACKGROUND AND PURPOSE: Contrast-enhanced 3D T1WI is a preferred sequence for brain tumor imaging despite the long scan time. This study investigated the clinical feasibility of ultrafast contrast-enhanced T1WI by 3D echo-planar imaging compared with a standard contrast-enhanced 3D MPRAGE sequence for evaluating intracranial enhancing lesions in oncology patients.
MATERIALS AND METHODS: Sixty-one patients in oncology underwent brain MR imaging including both contrast-enhanced T1WI, 3D-EPI and 3D MPRAGE, in a single examination session for evaluating intracranial tumors. Two neuroradiologists evaluated image quality, lesion conspicuity, diagnostic confidence, number and size of the lesions, and contrast-to-noise ratio measurements from the 2 different sequences.
RESULTS: Ultrafast 3D-EPI T1WI did not reveal significant differences in diagnostic confidence, contrast-to-noise ratiolesion/parenchyma, and the number of enhancing lesions compared with MPRAGE (P > .05). However, ultrafast 3D-EPI T1WI revealed inferior image quality, inferior anatomic delineation and greater susceptibility artifacts with fewer motion artifacts than images obtained with MPRAGE. The mean contrast-to-noise ratioWM/GM and visual conspicuity of the lesion on ultrafast 3D-EPI T1WI were lower than those of MPRAGE (P < .001).
CONCLUSIONS: Ultrafast 3D-EPI T1WI showed comparable diagnostic performance with sufficient image quality and a 7-fold reduction in scan time for evaluating intracranial enhancing lesions compared with standard MPRAGE, even though it was limited by an inferior image quality and frequent susceptibility artifacts. Therefore, we believe that ultrafast 3D-EPI T1WI may be a viable option in oncology patients prone to movement during imaging studies.
ABBREVIATIONS:
- CE
- contrast-enhanced
- CNR
- contrast-to-noise ratio
- ICC
- intraclass correlation coefficient
- SPACE
- sampling perfection with application-optimized contrasts by using different flip angle evolutions
Precise evaluation of intracranial malignancy is important in oncology patients for accurate staging and proper treatment planning.1⇓-3 Contrast-enhanced (CE) T1WI is an essential sequence in oncology patients used to evaluate malignant intracranial lesions, given its excellent capacity for soft-tissue contrast and contrast-enhancing effects following gadolinium injection.4⇓-6 In clinical practice, magnetization-prepared 3D gradient recalled-echo pulse sequences including MPRAGE, 3D turbo field echo, and brain volume imaging are widely used for evaluating brain tumors.7⇓-9 These 3D sequences are suitable for detecting small, enhancing lesions due to the high spatial resolution and 3D evaluation of tumor burden.10 However, high-resolution isotropic T1WI sequences usually require 2–5 minutes of scan time, and T1WI sequences are typically obtained twice, pre- and postgadolinium administration.11 This longer scan duration can be a major drawback for patients in oncology who do not tolerate long scan times due to poor general conditions.
Recently, Norbeck et al11 developed a rapid T1-weighted brain imaging sequence using a fat-suppressed multishot 3D-EPI, and this novel 3D-EPI sequence can be used to rapidly acquire isotropic T1-weighted volumes using a high phase-encoding bandwidth and radiofrequency pulses that reduce magnetization transfer effects. This study suggested the potential clinical application of ultrafast 3D-EPI T1WI to assess brain tumors with <30 seconds of scan time.11 However, the study did not fully evaluate the overall image quality or the diagnostic performance of this ultrafast 3D T1WI sequence, and too few cases were included to assess the clinical utility of the novel sequence. To the best of our knowledge, no previous studies have compared this novel ultrafast 3D-EPI T1WI sequence with the conventional 3D T1WI sequence for detecting intracranial lesions. Therefore, we investigated the clinical feasibility of the ultrafast 3D-EPI T1WI for evaluating intracranial enhancing lesions in oncology patients compared with conventional 3D T1WI, by assessing the overall image quality and diagnostic performance.
MATERIALS AND METHODS
Study Population
We retrospectively reviewed the medical records database of our institution and identified 61 patients who underwent diagnostic brain MR imaging, including CE ultrafast 3D-EPI T1WI and CE MPRAGE in a single session from August 2020 to February 2021. The 2 sets of CE 3D T1WI sequences were obtained to evaluate intracranial lesions in oncology patients with known or suspected intracranial tumors. The identified patients included 38 men and 23 women (age range, 19–81 years; mean age, 61 years). The indications for MR imaging were as follows: work-up or follow-up of brain metastasis (54/61, 88.5%) with known malignancies (lung cancer, 42; breast cancer, 9; prostate cancer, 2; and rectal cancer, 1) and follow-up of known primary brain tumors (7/61, 11.5%) (glioblastoma, 4; anaplastic oligodendroglioma, 1; anaplastic astrocytoma, 1; and brain stem glioma, 1).
Retrospective data collection and analysis were performed according to our institutional review board guidelines. This study was approved by the institutional review board at Gyeongsang National University Changwon Hospital. The institutional review board determined that patient approval and informed consent were not required for reviewing images and records due to the retrospective nature of the study.
Imaging Acquisition
MR imaging was performed using a 3T system (Signa Architect; GE Healthcare) with a 48-channel head coil. In addition to the postgadolinium ultrafast 3D-EPI T1WI and MPRAGE sequences, standard imaging sequences with axial T1WI, axial T2WI, axial FLAIR, and a 3D multiecho gradient-echo sequence (susceptibility-weighted angiography, ie, T2 star-weighted angiography) were acquired. Technical details of the MR imaging sequences are provided in the Online Supplemental Data. A dose of 0.2 mL/kg body weight of gadoteric acid (Dotarem 0.5 mmol/mL; Guerbet) was administered with an MR imaging–compatible power injector (MRXperion; Medrad Inc.), followed by a saline flush of 30 mL. The first postcontrast scan was started 2 minutes after injection of the contrast agent. At our institution, we use the ultrafast 3D-EPI T1WI for clinical purposes in patients restless during the scan, and the decision to acquire the ultrafast protocol images during examination is made by attending neuroradiologists during the day or supervising technologist at night or on holidays. The 2 different 3D T1WIs were obtained by inconsistent order with ultrafast 3D-EPI T1WI followed by MPRAGE in 29 patients and by reverse order in 32 patients.
Qualitative Radiologic Assessment
All data sets were anonymized and randomized. Two readers reviewed all images using a PACS and were blinded to the clinical diagnosis. However, readers were not blinded to the type of sequences due to the distinctive characteristic of the sequences. Two attending neuroradiologists with 11 and 6 years of experience performed an independent analysis of ultrafast 3D-EPI T1WI and MPRAGE to assess the overall image quality and diagnostic performance of the ultrafast 3D-EPI T1WI from a clinical feasibility perspective. Readers were instructed to report every intracranial contrast-enhancing lesion not assignable to the normal anatomic structure. When the reviewers detected an enhancing brain tumor, it was marked with an arrow on the captured images with the enhancing tumors. In case of disagreement between the 2 readers, a decision was made by consensus. Reconstructive images were provided in the axial, sagittal, and coronal planes with 1-mm section thickness. The score of each item for qualitative assessment was rated using a 5-point Likert scale12 as shown in the Online Supplemental Data.
Quantitative Radiologic Assessment
The readers drew an ROI in the largest enhancing lesion in each patient that was >0.5 cm in the largest diameter. Entirely necrotic or cystic lesions without a solid component were excluded from the contrast-to-noise (CNR) measurements because a suitable ROI could not be drawn. The CNR of contrast-enhancing brain lesions was estimated for both ultrafast 3D-EPI T1WI and MPRAGE using the following formula:
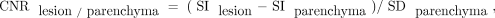 where signal intensity (SI) is the average signal intensity of the ROI, and SD is the SD of the ROI. SI and SD for the calculation of CNRlesion/parenchyma values were taken from the adjacent normal-appearing white matter and not from the image background. ROIs were carefully placed in the center of the enhancing lesion, avoiding tumor margins. ROIs of each 2 contrast-enhanced sequences were placed visually in a side-to-side comparison on 2 monitors using enlarged imaging data. The area of the ROI was dependent on the enhancing lesion size, varying between 20 and 42 mm2. The ROI from adjacent parenchyma measured 20 mm2. Furthermore, the CNR for the GM and WM differentiation was estimated using the following formula:
where signal intensity (SI) is the average signal intensity of the ROI, and SD is the SD of the ROI. SI and SD for the calculation of CNRlesion/parenchyma values were taken from the adjacent normal-appearing white matter and not from the image background. ROIs were carefully placed in the center of the enhancing lesion, avoiding tumor margins. ROIs of each 2 contrast-enhanced sequences were placed visually in a side-to-side comparison on 2 monitors using enlarged imaging data. The area of the ROI was dependent on the enhancing lesion size, varying between 20 and 42 mm2. The ROI from adjacent parenchyma measured 20 mm2. Furthermore, the CNR for the GM and WM differentiation was estimated using the following formula:

For the assessment of CNRWM/GM, ROIs were centrally placed in the splenium of the corpus callosum and in the left thalamus. Each ROI measured 20 mm2. The longest diameters of the largest enhancing lesion in each patient were measured in both ultrafast 3D-EPI T1WI and MPRAGE.
Statistical Analysis
The Kolmogorov-Smirnov test was used to test the distribution of variables. Normality was rejected for the number of lesions, size of the lesions, CNR WM/GM, and CNR lesion/parenchyma; thus, the Wilcoxon signed rank test was used to evaluate differences. The reader’s rating, lesion conspicuity, and diagnostic confidence were ordinal variables; thus, the Wilcoxon signed rank test was used. The intraclass correlation coefficient (ICC) was performed to assess the reliability of the number of lesions and the size of the lesions between the two 3D T1 sequences. We interpreted ICC values as follows: <0.5, poor reliability; 0.5–0.75, moderate reliability; 0.75–0.9, good reliability; and >0.9, excellent reliability.13
Interobserver agreement between the 2 readers was calculated by weighted κ statistics; 0–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.00 were interpreted as slight, fair, moderate, substantial, and almost perfect agreement, respectively, on the basis of the method of Landis and Koch.14
We performed all statistical analyses with MedCalc, Version 19.8 (MedCalc Software) and SPSS, Version 24.0 (IBM). P values < .05 were considered statistically significant.
RESULTS
Study Population
Of the 61 included patients, 36 (59.0%) presented with the following enhancing intracranial lesions: brain metastasis (22/36, 61.1%), glioblastoma multiforme (5/36, 13.9%), meningioma (3/36, 8.3%), anaplastic oligodendroglioma (1/36, 2.8%), anaplastic astrocytoma (1/36, 2.8%), cavernous sinus hemangioma (1/36, 2.8%), vestibular schwannoma (1/36, 2.8%), trigeminal schwannoma (1/36, 2.8%), and subacute infarction (1/36, 2.8%).
Diagnostic Performance of the Two 3D T1 Sequences
There was no statistically significant difference in terms of mean diagnostic confidence between the ultrafast 3D-EPI T1WI and MPRAGE (4.78 [SD, 0.48] versus 4.86 [SD, 0.35], P = .180). However, the mean visual conspicuity scores of contrast-enhancing lesions on ultrafast 3D-EPI T1WI were significantly inferior to those of MPRAGE (4.11 [SD, 0.40] versus 4.94 {SD, 0.23], P < .001), though the mean score of lesion conspicuity for the ultrafast 3D-EPI T1WI was>4 points and demonstrated well-depicted lesions with adequately visualized margins.
The number of enhancing lesions detected on the ultrafast 3D-EPI T1WI and MPRAGE did not show any significant differences (152 lesions versus 150 lesions; P = .577), and the results of the ICC demonstrated excellent agreement (0.998; 95% CI, 0.997–0.999). When we considered MPRAGE as a reference standard, 3 enhancing lesions on the ultrafast 3D-EPI T1WI were considered pseudolesions due to signal pileup by susceptibility artifacts from hemorrhage (Fig 1). In addition, in 1 patient, ultrafast 3D-EPI T1WI could not detect an enhancing lesion of the temporal lobe base because of susceptibility artifacts in the skull base (Fig 2), whereas there was a case of susceptibility artifacts in the temporal lobe base misinterpreted as a brain metastasis on ultrafast 3D-EPI T1WI (Fig 3).
A 63-year-old man who underwent brain MR imaging for metastasis work-up due to lung cancer. Upper row (A and B) displays CE ultrafast 3D-EPI T1WI; the lower row (C and D) displays standard MPRAGE. The enhancing hemorrhagic nodules in both parietal lobes are well-visualized on both ultrafast 3D-EPI T1WI and standard MPRAGE. However, the size of the enhancing portions is underestimated on ultrafast 3D-EPI T1WI due to susceptibility artifacts of the hemorrhagic component (arrows). Whereas eccentric hyperintensity of the hemorrhagic nodule in the right parietal lobe on ultrafast 3D-EPI T1WI was misinterpreted as an enhancing portion (arrowheads, A), there was no enhancing component on standard MPRAGE (arrowhead, C). Therefore, the eccentric hyperintensity (arrowheads, A) was considered as a pseudolesion due to signal pileup artifacts adjacent to the hemorrhagic portion.
A 73-year-old woman who underwent brain MR imaging for metastasis work-up due to lung cancer. Upper row (A–E) displays CE ultrafast 3D-EPI T1WI, and lower row (F–J) displays the standard MPRAGE. Multiple enhancing metastatic nodules are well-delineated on both ultrafast 3D-EPI T1WI and standard MPRAGE (arrows, A, B, F, G). An enhancing metastatic nodule in the right temporal lobe base was missed on ultrafast 3D-EPI T1WI by reviewers because it was considered a portion of a susceptibility artifact (arrowheads, C–E). The enhancing metastatic nodule is conspicuously delineated on standard MPRAGE (arrowheads, H–J). Pulsation artifacts of the basilar artery are shown as a hyperintense focus on ultrafast 3D-EPI T1WI (dashed arrow, C); however, the lesion was not interpreted as an enhancing nodule due to its characteristic location.
A 68-year-old man who underwent brain MR imaging for metastasis work-up due to lung cancer. Upper row (A–D) displays CE ultrafast 3D-EPI T1WI, and lower row (E–H) displays standard MPRAGE. Multiple enhancing nodules show that rim enhancement or nodular enhancement in both temporal lobes is well-visualized on both ultrafast 3D-EPI T1WI and standard MPRAGE (arrows, A, B, E, F). On ultrafast 3D-EPI T1WI, a suspicious rim-enhancing nodule was misinterpreted as metastasis by reviewers (arrowheads, B, C, D). However, compared with the standard MPRAGE images, the lesion was confirmed to be a pseudolesion due to susceptibility artifacts in the temporal lobe base.
There was no statistically significant difference with regard to the longest diameters of the largest enhancing lesions in each patient when comparing ultrafast 3D-EPI T1WI and MPRAGE (mean, 1.76 [SD, 1.16 ] versus 1.73 [SD, 1.12] cm; P = .180), and there was excellent agreement between the 2 different 3D T1WI sequences (ICC = 0.996; 95% CI, 0.993–0.998).
Image Quality Assessment
The image quality scores of the 2 readers and the corresponding interobserver reliability are shown in the Online Supplemental Data. Although the assessment of overall image quality and anatomic delineations on the ultrafast 3D-EPI T1WI showed significantly lower scores than those of MPRAGE (P < .001), the ultrafast 3D-EPI T1WI showed sufficient image quality, with >3 points on the average rating of the assessment. For the susceptibility artifacts, ultrafast 3D-EPI T1WI showed significantly more severe susceptibility artifacts compared with MPRAGE (P < .001); however, motion artifacts were significantly lower in ultrafast 3D-EPI T1WI than in MPRAGE (P < .001) (Online Supplemental Data). The interobserver agreement of the 2 readers showed moderate agreement in most items of image quality assessment, except for paradoxically low values due to the imbalanced number of concordant and discordant pairs.15,16
The mean value of CNRWM/GM was significantly lower for the ultrafast 3D-EPI T1WI than for MPRAGE (2.30 [SD, 1.76] versus 5.88 [SD, 2.00], respectively; P < .001). In addition, the mean value of CNRlesion/parenchyma of the ultrafast 3D-EPI T1WI was also lower than that of MPRAGE; however, there was no significant difference between the 2 sequences (16.07 [SD, 12.40] versus 22.45 [SD, 19.90]; P = .107).
DISCUSSION
In this study, we determined that the ultrafast 3D-EPI T1WI showed sufficient diagnostic image quality and comparable diagnostic performance for detecting enhancing intracranial lesions in oncology patients with fewer motion artifacts and a 7-fold reduction in scan time, compared with the standard MPRAGE sequence. Conversely, the ultrafast 3D-EPI T1WI had overall inferior image quality with more susceptibility artifacts and lower CNRGM/WM than the standard MPRAGE sequence.
For the assessment of brain metastasis, a 3D sequence has the advantage in detecting small, enhancing lesions due to the higher spatial resolution, which reduces the partial volume effects compared with 2D sequences.10 Additionally, a multiplanar reformation is another advantage of 3D sequences for assessing brain tumors within the complex brain anatomy.17 However, the relatively long scan time required for 3D sequences is a major drawback to applying this sequence in oncology patients presenting with a poor general condition and who cannot tolerate long scan times due to restlessness, which may contribute to increasing motion artifacts and patient anxiety.18,19 To date, a few studies have focused on reducing scan times without the loss of important clinical information from CE 3D T1 sequences for brain MR imaging protocols. In a recent study, ultrafast 3D-EPI T1WI was developed using a fat-suppressed multishot 3D-EPI to obtain isotropic T1-weighted volumes, revealing the possibility of clinical application of this novel sequence for brain imaging.11 However, to the best of our knowledge, there have been no studies comparing ultrafast 3D-EPI T1WI and conventional 3D T1 sequences to evaluate intracranial lesions by assessing the diagnostic performance and overall image quality from the perspective of the clinical application.
In this study, ultrafast 3D-EPI T1WI showed diagnostic performance comparable with that of the standard MPRAGE sequence. This result was similar to that of a recent study that showed equivalent diagnostic performance with only marginally higher background noise using a highly accelerated Wave-Controlled Aliasing in Parallel Imaging (Wave-CAIPI; Siemens Healthineers) 3D T1 sampling perfection with application-optimized contrasts by using different flip angle evolutions (SPACE) sequence for detecting brain metastasis at 3T.20 However, the total scan time of the accelerated Wave-CAIPI T1 SPACE sequence was 1 minute 40 seconds, which is >3 times longer than ultrafast 3D-EPI T1WI and is required at the expense of additional calibration and reconstruction effort. In contrast, the ultrafast 3D-EPI T1WI can provide comparable diagnostic confidence and CNRlesion/parenchyma in 30 seconds using the Cartesian acquisition scheme and the conventional parallel imaging to reduce anxiety and discomfort more efficiently for oncology patients in real-world practice. In particular, our results showed that the CNRlesion/parenchyma of the ultrafast 3D-EPI T1WI was not significantly different from that of the standard MPRAGE. This finding was consistent with the previous studies that proposed the clinical implication of CNRlesion/parenchyma to detect enhancing metastatic lesions.21⇓-23 We believe that this finding was valuable because CNRlesion/parenchyma is known as a key factor for contributing the higher detectability of enhancing lesions.22,23
In contrast, the overall image quality of ultrafast 3D-EPI T1WI in our study was considered inferior, compared with representative cases from the previous study,11 even though it is difficult to perform a direct comparison of our image quality with that of the original work at this time. The exact reasons for these differences are unclear, though these may be related to the intrinsic difference in the scanning environment, including the scanner and the number of shots of EPI between the 2 institutions. In general, any newly developed sequence should be validated in various ways to establish its clinical utility. From this perspective, we believe that our results are meaningful in that they can provide additional information regarding the acquisition of this novel sequence in different scan environments.
With regard to the technical aspects of ultrafast 3D-EPI T1WI, the inherently unavoidable geometric distortion of EPI-derived sequences cannot be completely removed at the air-tissue interface, even though this novel ultrafast 3D-EPI T1WI can further reduce the geometric distortion using the higher number of shots (here equal to 12) with the parallel imaging factor (here equal to 2) in contrast to the 2D-EPI sequence, which typically uses a single shot with parallel imaging.11 While the parallel imaging factor is coil-limited and may not be increased beyond 3 to avoid SNR loss, increasing the number of EPI shots reduces the geometric distortions at the expense of longer scan times. Ultimately, with the number of shots equal to the number of acquired lines, the 3D-EPI sequence will be distortion-free with the same scan time. We found that the current setting of 12 EPI shots gives a reasonable trade-off between scan time and geometric distortions because there was a minimal difference in the overall diagnostic accuracy. However, this sequence still presents the potential limitation relative to overall image quality, which can be degraded by susceptibility artifacts and results in insufficient detection of enhancing lesions near the skull base and brain stem. In addition to the lower SNR, this issue may also contribute to the concerns about the ultrafast 3D-EPI T1WI, such as inferior overall image quality and the lower mean value of the CNRWM/GM. Therefore, for the patient in whom the expected lesion is in the vicinity of a tissue-air interface (such as the pituitary gland), where the field inhomogeneity is high, one could increase the number of shots to obtain higher geometric accuracy at the expense of a longer scan time.
Furthermore, we observed hypointense or hyperintense dots in the pons, induced by pulsation artifacts of the basilar artery, which mimicked the small, enhancing lesions at first glance. However, the readers could easily distinguish these characteristic artifacts from pathologic conditions without any remarkable impact on decision-making in this study. It may be helpful to apply a sagittal scan plane with frequency-encoding in the superior-inferior direction to reduce the pulsation artifacts.7,11 In addition, the use of inferior saturation bands for the axial image acquisition is another possible option, but this use may significantly decrease the CNRWM/GM due to magnetization transfer effects.11 However, in the current study, we obtained the ultrafast 3D-EPI T1WI with a sagittal scan plane; thus, we did not compare the degree of pulsation artifacts directly between the sagittal and axial scan planes. In the present study, we also observed that accompanying artifacts in and around the hemorrhagic lesion may lead to difficulties in evaluating the enhancing lesion; in particular, susceptibility artifacts of hemorrhagic metastasis may result in the underestimation of the enhancing portion, and signal pileup in the vicinity of hemorrhagic lesions may mimic the enhancing component.11 Therefore, further technical advances are needed to correct the aforementioned issues before expanding the diagnostic use of ultrafast 3D-EPI T1WI in clinical practice.
Despite these shortcomings, ultrafast 3D-EPI T1WI can obtain CE 3D T1WI with a resolution of 1.2 × 1.2 × 1.2 mm3 with a short scan time of 30 seconds, which is approximately 7 times shorter than the standard MPRAGE sequence. In addition, the diagnostic confidence and CNRlesion/parenchyma of ultrafast 3D-EPI T1WI were comparable with those of standard MPRAGE. Therefore, ultrafast 3D-EPI T1WI can be used as a feasible alternative in certain clinical situations such as motion-prone patients or for unexpected termination of scans while obtaining conventional 3D T1WI.
This study has several limitations. First, there was an unavoidable selection bias because the data from all patients were evaluated retrospectively, the sample size was small, and the study was conducted in a single center. Second, we could not handle the acquisition order of the 2 different 3D T1WI sequences because of the retrospective study design. Thus, we did not consider the potential differences related to the timing bias between contrast injection and image acquisition, which can increase contrast agent uptake due to the delay. A future prospective study with a large study population is needed to validate the effect of the differences in postcontrast time delay. Third, we did not conduct the study including the patient groups with homogeneous types of brain tumors. In contrast, we took the pragmatic approach to obtain real-time data in daily clinical practice because this study was a feasibility study of the ultrafast 3D-EPI T1WI for identifying lesions in oncology patients. Therefore, it would not be necessary to provide additional analysis according to the specific pathologic diagnosis. Our results showed a clinically acceptable diagnostic image quality and lesion detectability with the benefit of a shorter scan time. Therefore, this broad study population can be helpful to generalize the clinical utility of ultrafast 3D-EPI T1WI to various types of brain tumors. Fourth, pathologic confirmation was not obtained for all brain tumors because patients with multiple brain tumors such as metastases usually do not undergo surgical intervention. Last, we did not apply the recommended time delay for obtaining the enhanced T1WI in brain tumor imaging.8,9 Even though previous studies have provided a recommendation for a brain MR imaging protocol in oncology patients,8,9 there is often a gap between real-world clinical practice and ideals. For practical reasons, it is believed that many previous studies did not accurately specify the delayed time or apply a delay time of <4 minutes. In this study, it was also difficult to apply the recommended protocol for enhanced T1WI in the study patients because actual clinical situations such as the number of MR imaging systems or the time table of the MR imaging room were inevitably considered.
CONCLUSIONS
We demonstrated that ultrafast 3D-EPI T1WI had a comparable diagnostic performance with sufficient image quality and 7-fold reduction in scan time for evaluating intracranial enhancing lesions in oncology patients compared with the standard MPRAGE sequence, even though it had minor issues due to an inherent geometric distortion. Therefore, we believe that ultrafast 3D-EPI T1WI may be a viable option that can be used clinically in lieu of the conventional 3D T1WI or as a backup sequence in specific clinical situations for oncology patients who cannot tolerate long scan times. Our results should be considered in the technical development of ultrafast 3D-EPI T1WI, and future studies with various clinical scenarios are needed to validate our results and help expand the clinical use of ultrafast 3D-EPI T1WI in daily practice.
Acknowledgments
We would like to thank our colleagues in the MR imaging section, Seong Jin Kim, Myungwook Lee, and Jae Hyun Lim, for their effort in ensuring that all MR images were obtained optimally, and we also thank Tae Byeong Kim for his help in strengthening our work.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received August 21, 2021.
- Accepted after revision October 29, 2021.
- © 2022 by American Journal of Neuroradiology















