Abstract
SUMMARY: Primary progressive aphasia is a clinically and neuropathologically heterogeneous group of progressive neurodegenerative disorders, characterized by language-predominant impairment and commonly associated with atrophy of the dominant language hemisphere. While this clinical entity has been recognized dating back to the 19th century, important advances have been made in defining our current understanding of primary progressive aphasia, with 3 recognized subtypes to date: logopenic variant, semantic variant, and nonfluent/agrammatic variant. Given the ongoing progress in our understanding of the neurobiology and genomics of these rare neurodegenerative conditions, accurate imaging diagnoses are of the utmost importance and carry implications for future therapeutic triaging. This review covers the diverse spectrum of primary progressive aphasia and its multimodal imaging features, including structural, functional, and molecular neuroimaging findings; it also highlights currently recognized diagnostic criteria, clinical presentations, histopathologic biomarkers, and treatment options of these 3 primary progressive aphasia subtypes.
ABBREVIATIONS:
- AD
- Alzheimer disease
- ASL
- arterial spin-labeling
- FTLD
- frontotemporal lobar degeneration
- lvPPA
- logopenic variant PPA
- nfvPPA
- nonfluent/agrammatic variant PPA
- PPA
- primary progressive aphasia
- 3R
- 3-repeat
- 4R
- 4-repeat
- svPPA
- semantic variant PPA
- TDP-43
- transactive-response DNA-binding protein 43
- TSPO
- translocator protein 18 kDa
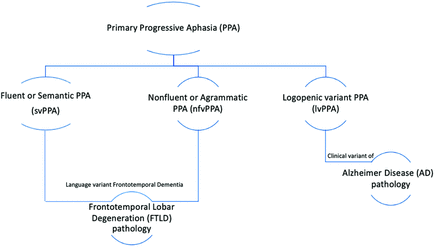
Neurodegenerative disorders encompass an assortment of clinically and histopathologically diverse conditions, typically slowly progressive and associated with gradual neurologic dysfunction. While precise mechanisms leading to their development continue to be elucidated, these disorders can be broadly grouped into categories based on similar histopathology, including tauopathies (eg, some cases of frontotemporal lobar degeneration [FTLD], corticobasal degeneration, progressive supranuclear palsy), transactive-response DNA-binding protein 43 (TDP-43) accumulation (eg, other cases of FTLD, amyotrophic lateral sclerosis), β-amyloid accumulation (eg, Alzheimer disease [AD]), and α-synucleinopathies (eg, Parkinson disease, dementia with Lewy bodies, multiple system atrophy), noting mixed pathologies in some cases.1 Primary progressive aphasia (PPA), clinically characterized by language-predominant impairment, has been histopathologically linked to both underlying FTLD (eg, semantic variant PPA [svPPA] and nonfluent/agrammatic variant PPA [nfvPPA]) and AD-type pathology2 (eg, logopenic variant PPA [lvPPA]) (Fig 1). The microtubule-associated protein τ, encoded by the MAPT gene, has been shown to pathologically aggregate when in an abnormal hyperphosphorylated form (p-τ) and result in extracellular neurofibrillary tangles that contribute to neurodegeneration. Tauopathies are characterized by the predominance of abnormally deposited alternatively spliced τ isoforms (eg, 3-repeat [3R] versus 4-repeat [4R]-tauopathies), which may be seen in some cases of PPA.3 Deposits of TDP-43, a cellular protein encoded by the TARDBP gene and with 4 described subtypes (A, B, C, D), is an additional frequently identified pathologic substrate seen in cases of PPA.4 While trends have been reported between these histopathologic entities and PPA subtypes (lvPPA: AD pathology; svPPA: TDP-43; nfvPPA: 4R-τ), no absolute association exists and inconsistencies regarding the frequency of molecular alterations for each variant have been described across studies, likely related to varied diagnostic approaches.5
PPA is divided into 3 recognized variants: svPPA, nfvPPA, and lvPPA. svPPA and nfvPPA exhibit FTLD pathology; svPPA and nfvPPA are considered to be language-variant frontotemporal dementias. lvPPA is a clinical variant of AD.
While a progressive neurodegenerative disorder characterized by language-predominant impairment has been a recognized entity dating back to the 19th century, inconsistencies regarding its terminology and nomenclature exist in the literature. In an effort to improve diagnostic uniformity and the reliability of research results, Gorno-Tempini et al6 published a 2011 framework for PPA diagnosis and classification using a 2-step process. Initially, patients must meet the criteria of Mesulam7,8 for the overarching diagnosis of PPA, requiring a language-predominant deficit in the absence of significant episodic memory, behavioral, or visuospatial disturbances,6 and are subsequently subdivided into the clinical variant that best corresponds to their specific language disturbance. Inclusion and exclusion criteria for the diagnosis of PPA, as well as the specific diagnostic criteria for each PPA subtype, are outlined in Tables 1 and 2. In addition to clinical features, a PPA diagnosis can be further supported by pathology (eg, FTLD- versus AD-type pathology) if such pathologic or genetic data are available as well as imaging if a typical pattern of atrophy and/or hypometabolism is identified. Therefore, an accurate diagnosis of PPA requires a multidisciplinary approach, inclusive of clinical, pathologic, and radiologic evaluation. Early in the disease course, some patients may be referred to as “PPA unclassifiable,” with further elucidation of their specific subtype as the disease progresses.6 While most patients do not have abnormalities on a general neurologic examination at diagnosis, features such as parkinsonism, apraxia, and upper motor neuron involvement have been reported as PPA progresses.9
Inclusion and exclusion criteria for PPA diagnosis
Diagnostic criteria for PPA variants
Advances in the understanding of PPA, particularly the recognition of variant-specific patterns of atrophy and/or hypometabolism, have reinforced the role of structural, functional, and molecular neuroimaging in supporting the diagnosis, when clinically suspected. This review highlights available imaging modalities in the identification of PPA as well as characteristic variant-specific features with which neuroradiologists should be familiar to aid in a prompt and accurate diagnosis.
Logopenic Variant PPA
lvPPA is the most recently described of the 3 PPA variants, first reported in 2004 by Gorno-Tempini et al.10 Clinically characterized by word-finding difficulties and lapses in conversation, lvPPA has been reported to exhibit histopathologic findings and biomarkers that overlap with AD pathology (eg, β-amyloid [A β] and neurofibrillary tangles)2 and, therefore, is considered a clinical variant of AD.
Classically, patients with lvPPA present with word-finding difficulties and lapses in conversation, giving rise to its “logopenic” name (Greek, “lack of words).11 Early in the disease, patients exhibit “tip-of-the-tongue” hesitation with pauses in word retrieval as well as anomia.4 Spelling or speech-sounding errors are also frequently described, and patients may struggle to understand complex sentences or retain verbal information. The diagnostic feature that distinguishes lvPPA from svPPA and nfvPPA is an early and disproportionate difficulty in repeating heard phrases and sentences, corresponding to an impairment of phonologic or verbal working memory.12 While language is the dominant issue, extralinguistic difficulties related to memory, praxis, and visuospatial awareness have been reported.13 Patients often exhibit generalized anxiety, irritability, and dependence on their primary caregivers, behavioral features that may also occur with typical AD or other AD variants.
As a whole, PPA is a rare entity with an estimated prevalence of 3–7 cases per 100,000, often occurring in late middle life (mean age of disease onset, 62.34 years) with an average delay between first symptoms and diagnosis of 3.21 years in 1 study.14 Given the novelty of lvPPA as a clinical entity, its precise prevalence is not definitively known. However, a 2016 retrospective analysis of a cohort from a tertiary center (n = 97) in patients with language deficits and CSF biomarkers from the French AD databank, performed to better understand PPA demographics, revealed lvPPA as the most common variant of PPA (51%, 49/97) with a slight female predominance (57%, 28:21, female/male). Within this group, lvPPA was more frequently associated with an AD CSF profile (85%) than nfvPPA (35%) or svPPA (20%), contributing to the present day notion of lvPPA as a clinical variant of AD.14
In a study investigating CSF fluid biomarkers (including A β 42, τ, p-τ) in 13 patients with lvPPA, 62% (8 of 13) demonstrated a profile indicative of AD pathology (lvPPA+), while 38% (5 of 13) had a non-AD profile (lvPPA–).15 Subsequent analyses demonstrated that those in the lvPPA+ group exhibited more advanced imaging findings compared with those in the lvPPA– group, including more extensive hypometabolism and larger regions of involvement throughout the inferior parietal and superior and middle temporal cortices. Such heterogeneity of pathologies identified in this study may reflect a “logopenic aphasia complex,” with at least 2 existing lvPPA subvariants.15
PPA may be inherited in an autosomal dominant manner, most commonly associated with mutations in the progranulin (GRN) gene on chromosome 17.6 While the presence of a GRN mutation does not necessarily lead to PPA, a language disorder often emerges in many patients with this mutation.12 The clinical symptoms of this language disorder can vary widely, with heterogeneity even among family members with the same GRN mutation. However, among patients with nonamyloid PPA with GRN mutations, lvPPA was found to be the most frequent linguistic variant.16 In 1 study, 42% of patients with lvPPA were found to carry the apolipoprotein E ε4 allele, known to confer an increased risk of sporadic AD and in keeping with our understanding of lvPPA as a clinical variant of AD, compared with 26% of patients with svPPA and 20% of patients with nfvPPA.17 Other predisposing gene variants or mutations found to be associated with lvPPA include TREM2, TOMM40, APP, PS1 and PS2, and MAPT.18
While the aforementioned evidence supports underlying AD pathology in most patients with lvPPA, not all cases of lvPPA are attributed to AD pathology. In 1 postmortem analysis of 99 patients with lvPPA, 76% had primary AD pathology, while FTLD-TAR DNA-binding protein (FTLD-TDP) and FTLD-τ pathologies were identified in 14% and 5% of patients, respectively.17
To date, no pharmacologic options have been shown to improve or protect against declining function in lvPPA. However, supportive care measures such as speech-language therapy have demonstrated efficacy in improving communication.19 Given that underlying AD pathology is associated with most lvPPA cases, use of emerging anti-amyloid therapies in lvPPA may be investigated.
Structural Imaging.
The presence of specific regional patterns of atrophy or metabolic impairment is the key neuroimaging diagnostic feature for each of the 3 PPA variants (Table 2). Structural imaging, including CT and MR imaging, can be used to identify these classic patterns of focal atrophy. Included in the 2011 Gorno-Tempini et al6 diagnostic criteria, MR imaging can be used to identify asymmetric, classically left-sided widening of the Sylvian fissure, indicative of the posterior peri-Sylvian and temporoparietal atrophy seen in lvPPA. The posterior aspect of the left superior temporal gyrus, corresponding to the expected Wernicke area, is typically involved. This finding, particularly when progressive over multiple examinations and in conjunction with a clinical history of progressive word-finding difficulty, should raise the possibility of underlying lvPPA (Fig 2). Notably, while most patients are left-hemispheric language dominant, involvement of the right hemisphere has been reported and hypothesized to occur in left-handed individuals or those with a history of developmental learning disabilities (eg, dyslexia).20
Coronal T1-weighted MR imaging (A), axial T1-weighted MR imaging (B), and sagittal T1-weighted MR imaging (C) in a right-handed individual with impaired repetition of phrases demonstrate asymmetric widening of the left Sylvian fissure with left posterior peri-Sylvian and temporoparietal atrophy (white arrows, A–C), suspicious for lvPPA.
The preferred structural imaging technique for the diagnosis of PPA is MR imaging, due to its superior soft-tissue resolution and ability to precisely localize anatomic atrophy. Advanced MR imaging techniques, such as voxel-based morphometry analysis and DTI, may be used to demonstrate focal GM atrophy (Fig 3) and WM alterations, respectively.21 In particular, cortical volumetric software such as the FDA-approved NeuroQuant (https://www.cortechs.ai/products/neuroquant-ct/) and Icometrix (https://www.icometrix.com/) are increasingly being used in routine clinical assessment of various neurodegenerative disorders. In a study investigating the utility of MR imaging in differentiating PPA variants, MR imaging demonstrated a high specificity for the characteristic atrophy patterns of lvPPA (95%) and nfvPPA (91%), noting a low sensitivity for both (43% for lvPPA; 21% for nfvPPA).22 Therefore, while the presence of left posterior peri-Sylvian or temporoparietal region atrophy is highly suggestive of lvPPA, its absence does not exclude the diagnosis. In a prospective study investigating 130 patients with neurodegenerative aphasia, of whom 52 had lvPPA, GM loss was identified in patients with lvPPA, more commonly on the left and greatest in the posterior temporal lobe extending to the frontal and parietal regions.23 Fractional anisotropy and mean diffusivity analyses within this cohort revealed left-greater-than-right bilateral WM involvement, greatest in the posterior left temporal WM and extending into the anterior temporal, frontal, parietal, and occipital WM, as well as involving the bilateral superior and inferior longitudinal fasciculi and inferior occipitofrontal fasciculus. The posterior superior temporal and inferior parietal cortices have been shown to play a role in phonologic loop functions.21 Therefore, involvement of these regions and WM tracts in the superior and inferior longitudinal fasciculi likely account for the poor repetition, naming, and comprehension seen in patients with lvPPA.23,24
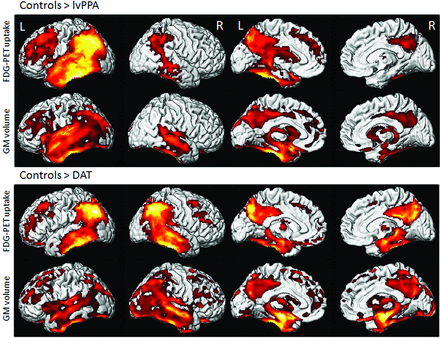
Voxel-level imaging findings in lvPPA and dementia of the Alzheimer type (DAT) compared with controls. 3D renderings show regions of reduced FDG metabolism and GM volume in lvPPA compared with controls and in DAT compared with controls. Note, lvPPA demonstrates hypometabolism and focal atrophy primarily in the left lateral temporal and inferior parietal lobes (including the left angular and supramarginal gyri) and left precuneus and left posterior cingulate gyrus. Adapted with permission from Madhavan et al.84 R indicates right; L, left.
Atrophy may also be identified anteriorly with involvement of the hippocampi, among other structures, with the overall extent and pattern of atrophy varying widely among individual patients. Most important, the presence of progressive atrophy with time supports the diagnosis of PPA, a critical observation worthy of mention when interpreting such structural imaging examinations.
Functional Imaging.
fMRI, which can be performed with task-based paradigms or in a resting state, uses blood oxygen level–dependent contrast to identify areas of brain activation on the basis of oxygen extraction.25 While fMRI is not in routine clinical use for the diagnosis of PPA and limited literature exists regarding its specific findings in lvPPA, fMRI has been reported to demonstrate functional changes in patients with svPPA (formerly referred to as semantic dementia),26 to be discussed later in this review. Such advanced imaging techniques could be useful in identifying aberrant and compensatory language network changes and may prove useful in guiding future therapeutic trials.11
Cerebral perfusion, which has been linked to cognition and neuronal activity, can be measured with MR imaging using the noncontrast arterial spin-labeling (ASL) technique.27 While limited data exist regarding use of ASL for lvPPA specifically, ASL has been shown to identify hypoperfusion patterns in other dementia subtypes before symptom onset and also correspond to disease-specific regions of hypometabolism identified on [18F] FDG-PET. However, limitations of the ASL technique for clinical use include its low SNR on conventional 1.5T field strength MR imaging, with 3T field strength MR imaging preferred for use in the ASL technique, as well as technical issues with quantification. Further studies assessing the potential role of ASL for patients with lvPPA as well as its performance compared with [18F] FDG-PET are warranted.
Molecular Imaging
Molecular imaging allows in vivo identification and quantification of cerebral metabolism, abnormal deposition of β-amyloid and τ, and the presence of brain inflammation, important neuroimaging biomarkers that may improve early diagnosis and assist in assessing neurodegenerative disease progression.27-29 Currently available molecular imaging modalities include SPECT and PET, with a number of investigational radiotracers on the horizon.30 Advantages of PET imaging include superior spatial and contrast resolution compared with SPECT, though it is a more expensive examination and less widely available. Limitations include attenuation correction and motion artifacts, which may cause inaccurate anatomic coregistration. Molecular neuroimaging has been shown to be useful in the diagnosis of PPA.31-33
[18F] FDG-PET assesses cerebral glucose metabolism, which is abnormally reduced in neurodegeneration due to synaptic dysfunction and neuronal loss.2 The characteristic pattern of hypometabolism in lvPPA includes asymmetric involvement of the left posterior peri-Sylvian and left lateral temporoparietal regions, mirroring previously described regions of atrophy, specifically involving the left inferior parietal lobule and left posterior superior and middle temporal gyri, including the expected Wernicke area (Broadman area 22) (Figs 3 and 4). SPECT, which demonstrates regional hypoperfusion similar to the metabolic alterations on [18F] FDG-PET is infrequently used in clinical practice due to technical disadvantages and poorer accuracy.29
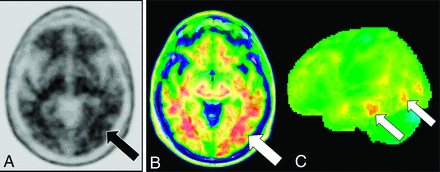
[18F] FDG-PET cortical surface maps demonstrate an abnormal FDG distribution pattern with moderate-to-severe hypometabolism in the left, lateral temporoparietal lobes including in the left precuneus and posterior cingulate gyrus (A), with corresponding disproportionate cortical atrophy in the lateral left temporoparietal region visualized on brain CT (B), findings are further supported by a semiquantitative FDG-PET analysis using z scores calculated in comparison with age-matched cognitively healthy controls, demonstrating markedly decreased values in the left parietal and left lateral temporal regions, including in the precuneus and posterior cingulate gyrus (B).
Amyloid PET is a valuable examination for the diagnosis of AD and other neurodegenerative disorders demonstrating A β-pathology.16 In a meta-analysis of 1251 patients from 36 dementia centers, A β-positivity was identified in 86% of patients with lvPPA with evidence of AD pathology identified in 76% of those who underwent a postmortem examination (Fig 5).17 A β-positivity was also seen in a minority of those with nfvPPA (20%) and svPPA (16%); however, A β-positivity was thought to represent a concomitant age-related process in these patients rather than being attributable to their PPA syndrome. In another study, 88% (46 of 52 patients) meeting the criteria for lvPPA demonstrated A β-positivity, with low A β-positivity rates in patients not meeting criteria for lvPPA (10%, 13 of 130 patients).18 In a study evaluating amyloid metabolism in PPA, 100% (4/4) of patients with lvPPA demonstrated elevated cortical Pittsburgh compound B uptake versus 16% (1/6) of patients with nfvPPA and 20% (1/5) of those with svPPA.19 Thus, amyloid PET imaging can be useful in distinguishing lvPPA from nfvPPA and svPPA, noting that comorbid age-related A βpathology may occur in each of these entities and an amyloid PET scan with positive findings does not equate to a diagnosis of lvPPA.
[18F] florbetaben PET axial gray-scale (A), axial color map fused to a T1-weighted MR image (B), and left lateral 3D stereotactic surface projection (C) demonstrate focal areas of increased cortical β-amyloid deposition in the left temporal lobe (arrows).
In addition to β-amyloid plaques, τ neurofibrillary tangles are a hallmark pathologic finding in AD.20 τ-targeting PET tracers have been used for molecular imaging in PPA, most commonly [18F] flortaucipir (AV-1451), an FDA-approved, first-generation τ PET ligand.21,22 In a study investigating use of [18F] AV-1451 in PPA, patients with lvPPA exhibited striking uptake throughout the neocortex, most notably in the left temporoparietal region, compared with controls and subjects with other PPA variants, confirming the use of this radiotracer in distinguishing PPA subtypes.20 In a case series of patients with typical amnestic AD and atypical variants (posterior cortical atrophy, lvPPA, and corticobasal syndrome), all patients demonstrated region-specific distribution of [18F] AV-1451, indicating that τ PET can serve as a key biomarker linking molecular AD neuropathologic conditions with clinically significant neurodegenerative syndromes.23
The use of PET tracers that target the translocator protein 18 kDa (TSPO) have also been explored in PPA, with the goal of characterizing the role of microglial activation and associated neuroinflammation in the pathogenesis of PPA. TSPO, originally named the peripheral benzodiazepine receptor, is an 18-kDa outer mitochondrial membrane protein, which has been found in disease-relevant areas across a broad spectrum of neurodegenerative diseases.24 Histopathologic studies have demonstrated asymmetric distribution of activated microglia in PPA, including high microglial densities in the superior temporal and inferior frontal gyri of the language-dominant hemisphere, consistent with postmortem and/or in vivo atrophy distribution.25 Patterns of microglial activation revealed variation favoring areas of increased atrophy in regions associated with language function, demonstrating concordance among patterns of microglial activation, atrophy, and clinical PPA phenotype.25 These findings support the potential use of TSPO PET in the evaluation of PPA subtypes.
Semantic Variant PPA
svPPA, previously referred to as “semantic dementia” in case reports throughout the 20th century, is a heterogeneous clinical syndrome characterized by progressive loss of knowledge about words and objects, including a fluent dysphasia with severe anomia, reduced vocabulary, and prominent impairment of single-word comprehension, which progresses to a stage of virtually complete dissolution of the semantic components of language.26 The criteria for the clinical diagnosis of svPPA are detailed in Table 2. Notably, a right temporal lobe–predominant variant of svPPA featuring impaired facial recognition (ie, prosopagnosia) and changes in affect and social behavior, in addition to semantic memory impairment, has also been described.34
SvPPA is currently recognized within the clinical spectrum of FTLD (Fig 1), with underlying histopathology most often attributable to TDP-43 type C, characterized by long dystrophic neurites and identified in >80% of patients with svPPA.5.35,36 Some cases have been associated with other histopathologic findings, including FTLD-TDP types A and B, FTLD-τ (particularly 3R-τ [Pick bodies] and 4R globular glial tauopathy), as well as AD pathology.5,35,36 SvPPA is almost always sporadic, rather than familial, and is the least heritable compared with other FTLD syndromes, with only 2%–4% of cases demonstrating an autosomal dominant pattern and suspected family history identified in 2%–17% of patients.26,37,38 Genetic disorders linked to familial FTLD-TDP include those associated with mutations in the GRN gene (FTLD-TDP type A), expansions in the chromosome 9 open reading frame 72 (C9orf72) gene (FTLD-TDP types A or B), and mutations in the valosin-containing protein (VCP) gene (FTLD-type D).39 In the absence of a strong family history of svPPA, an underlying genetic abnormality is considered unlikely.35
The precise prevalence of svPPA is not definitively known because diagnosis requires extensive clinical expertise and available data has largely been derived from tertiary care research center referrals, likely not representative of the general population. However, the 2016 retrospective analysis from the French AD databank identified 26% (25 of 97) of PPA cases with CSF biomarkers to be svPPA, with a mean age of disease onset at 59.5 years and an average delay of 4.5 years between first symptom and diagnosis, a male predominance (68%; 8:17, female/male), and underlying AD pathology in only 20% of cases.14 The estimated svPPA prevalence based on the French AD databank cohort was 0.8 per 100,000 individuals, increasing with patient age. With regard to FTLD, its overall prevalence varies widely across studies, ranging from 2 to 31 per 100,000 individuals with an estimated true point prevalence of 15 to 22 per 100,000 and an incidence of 2.7 to 4.1 per 100,000 in those younger than 70 years of age.40 One series assessing 353 consecutive patients with FTLD identified 18.7% as having svPPA.41 An epidemiologic study investigating FTLD syndromes in 2 UK counties with a population of 1.69 million yielded an estimated svPPA prevalence of 1.2 per 100,000.42
No disease-modifying medications are currently available for the treatment of svPPA. A variety of psychotropic medications have been used to manage associated behavioral symptoms, though evidence of efficacy from randomized clinical trials is lacking. Speech-language therapy has been shown to slow progression of anomia and may even offer a protective benefit to lexical items not yet lost, noting that treatment is suspected to be most beneficial at early stages of disease, supporting the advantage of an early diagnosis.43 A double-blind, sham-controlled, randomized clinical trial of transcranial direct current stimulation in patients with svPPA is ongoing.44
Structural Imaging.
The most common imaging features associated with svPPA include regional atrophy predominantly involving the left temporal lobe, most marked anteriorly involving the temporal pole (Fig 6).45 While focal atrophy is typically more pronounced on the left side, patients presenting with right-dominant temporal atrophy have been described in the literature (Fig 7).34,46 A study involving voxel-based morphometry in patients with svPPA reported that those with prosopagnosia had bilateral temporal lobe GM volume loss with greater involvement on the right, while those without prosopagnosia had predominantly left anterior temporal lobe volume loss.47
Coronal T1-weighted MR imaging (A), axial T1-weighted MR imaging (B), and axial T2-weighted MR imaging (C) in a right-handed individual with impaired single-word comprehension demonstrate marked asymmetric atrophy of the anterior left temporal lobe (white arrows, A–C), suspicious for svPPA.
Axial T1-weighted MR imaging (A) and axial and coronal T1-weighted MR imaging fused with [18F] FDG-PET (B and C) in a left-handed individual with impaired single-word comprehension demonstrate marked asymmetric atrophy of the anterior right temporal lobe (black arrow, A) with corresponding marked hypometabolism (white asterisks, B and C) due to svPPA.
A meta-analysis of voxel-based morphometry studies investigating svPPA identified reduced GM volume in the bilateral fusiform and inferior temporal gyri, extending to the medial portion of the temporal lobes with involvement of the amygdala and parahippocampal gyri, as well as the left temporal pole, middle temporal gyrus, and caudate nucleus.48 Surface-based analysis of patients with svPPA identified marked cortical thinning in the left temporal lobe, particularly at the temporal pole; entorhinal cortex; and parahippocampal, fusiform, and inferior temporal gyri, with similar-yet-less extensive involvement of the contralateral cortex.49 Similarly, a longitudinal investigation mapping the progression of GM atrophy in predominantly left-versus-predominantly right temporal lobe variants of svPPA identified significant progression of GM atrophy in both the affected and contralateral temporal regions.50 Voxel-based morphometry has also identified asymmetric regional reduction in the temporal, periventricular, and callosal WM in patients with svPPA.51
DTI has been used to study structural connectivity changes on the whole-brain level in patients with svPPA, with reports of reduced fractional anisotropy and increased diffusivity in the anterior temporal lobe extending dorsally and posteriorly into the ventral frontal regions.52 Tractography has similarly been implemented in svPPA, identifying disruptions of structural connectivity related to GM atrophy, most severely affecting the WM tracts connecting the temporal regions with the frontal, parietal, and occipital regions (ie, uncinate fasciculus, arcuate fasciculus, superior longitudinal fasciculus, and inferior longitudinal fasciculus).52
Functional Imaging.
Task-based fMRI studies have reported that patients with svPPA compared with healthy controls demonstrate decreased activation in the mid-fusiform and superior temporal gyri; increased activation in the intraparietal sulcus, inferior frontal gyrus, and left superior temporal gyrus/sulcus; and a lack of activation in the anterior temporal lobe.35,52 Similarly, resting-state fMRI studies in patients with svPPA have demonstrated reduced functional connectivity in the language and executive networks, with extensive disruptions between the anterior temporal lobe and a broad range of brain regions across the temporal, frontal, parietal, and occipital lobes.35,52,53 Magnetoencephalographic imaging has also been implemented to investigate whole-brain resting-state functional connectivity, identifying significant hyposynchrony of α and β frequencies within the left temporoparietal junction in patients with svPPA.54
Regarding ASL MR imaging, a study investigating the prognostic value of regional CBF as measured by ASL MR imaging in patients with svPPA reported that ASL MR imaging may be sensitive to functional changes not identified on structural MR imaging, potentially serving as a prognostic biomarker marker of disease progression.55 Further studies assessing the potential role of ASL in patients with svPPA as well as its performance compared with [18F] FDG-PET are warranted.
Molecular Imaging.
[18F] FDG-PET and SPECT can be performed to demonstrate characteristic asymmetric hypometabolism/hypoperfusion predominantly affecting the anterior temporal regions, most commonly involving the left temporal pole (Figs 8 and 9). While svPPA is often associated with TDP-43 pathology, no available PET radioligand for TDP-43 exists to date.
[18F] FDG-PET (A), axial T1 (B), and PET MR imaging (C) views demonstrate an abnormal FDG distribution pattern with markedly decreased tracer uptake in the temporal lobes, particularly in the left temporal pole. There is corresponding advanced cortical atrophy with a “knife-blade” appearance in the left anterior temporal lobe on the axial T1 sequence.
[18F] FDG-PET cortical surface maps demonstrate an abnormal FDG distribution pattern with severe left and moderate right hypometabolism in the anterior temporal lobes (A), with corresponding disproportionate cortical atrophy, particularly pronounced in the left temporal pole visualized on brain CT images (B), findings further supported by semiquantitative FDG-PET analysis using z scores calculated in comparison with findings in age-matched cognitively healthy controls, semiquantitative FDG-PET analysis demonstrate markedly decreased values in the temporal lobes including the temporal poles (left > right) (B).
Given the presence of AD pathology in some cases of svPPA, reported in up to 20% of cases within the 2016 French AD databank retrospective cohort,14 amyloid PET may result in an amyloid-positive examination in a minority of svPPA patients.
While τ pathology is infrequently reported in patients with svPPA, studies with radioligands that demonstrate an affinity for τ, including [18F] THK-5351 and flortaucipir ([18F] AV-1451), have demonstrated asymmetric retention in the temporal lobes in a pattern consistent with the expected distribution of TDP-43 pathology, hypothesized to reflect off-target binding to monoamine oxidase as a consequence of inflammation in the affected regions.21,56-58 A study investigating the novel τ PET tracer [18F] PI-2620, which has a low affinity for monoamine oxidase, demonstrated slightly elevated uptake involving the anterior and lateral temporal lobes in 1 of 2 subjects with svPPA, without elevated uptake in the other subject [Fig 10].59 There are no studies to date of PET ligands with an for inflammatory biomarkers (eg, monoamine oxidase B, TSPO/peripheral benzodiazepine receptor, or cyclooxygenase) in svPPA.60
Fused [18F] PI-2620 τ-PET and T1 MPRAGE MR imaging from subjects 66 (A) and 78 years of age (B) with semantic PPA. Notably, [18F] PI-2620 has a low affinity for monoamine oxidase, and subject A demonstrates no focal increased tracer uptake. However, subject B shows binding spanning the anterior and lateral temporal lobes (left greater than right) with corresponding atrophy on MR imaging. Adapted with permission from Mormino et al.59
Nonfluent/Agrammatic PPA
nfvPPA, previously referred to as “progressive nonfluent aphasia” in a series by Grossman et al61 and “PPA with agrammatism” by Mesulam,62 is the most diverse of the 3 PPA subtypes. Classically, patients present with a progressive language-predominant disturbance characterized by agrammatism in language production and apraxia of speech, with abnormally short (ie, telegraphic) phrases that tend to lack function words.62 Patients with nfvPPA have reduced verb production and diminished complexity in terms of grammar use,63 as well as difficulty with complex coordination of muscle groups involved in articulation of speech sounds (ie, apraxia of speech) and distortion of prosody (ie, rhythm, stress, and intonation of speech).64 The rate of word production in patients with nfvPPA has been reported to be less than one-third of the rate in healthy adults.63 Single-word comprehension is notably preserved, though patients with nfvPPA may experience difficulty understanding sentences with complex syntax, such as those with relative clauses (eg, “he met a man who knew his brother”) or those with passive voice (eg, “the snake was bitten by the mongoose”).62,64 Complete criteria for a clinical diagnosis of nfvPPA, as defined by Gorno-Tempini et al,6 are described in Table 2.
Although typically considered a tauopathy, nfvPPA is the most heterogeneous of the PPAs, with a variety of other associated underlying pathologies. While 4R-τ is the most commonly reported underlying pathology, 1 postmortem series identified 23% of patients with nfvPPA exhibiting 3Ra-τ pathology (Pick bodies) and a minority with underlying TDP-43 or AD-type pathology.65 Patients with apraxia of speech and parkinsonism are more often associated with having a tauopathy than TDP-43 pathology.5,66 Similar to its pathologic heterogeneity, the clinical spectrum of nfvPPA has been described as the most diverse of the PPA subtypes, with a number of variant nfvPPA subsyndromes reported.11
NfvPPA is also the most heritable of the PPAs, with approximately 30% of patients reporting a positive family history.67 Mutations in all of the major genes associated with FTLD (eg, GRN, MAPT, C9orf72) have been identified in nfvPPA. Therefore, genetic screening should be considered in patients with a relevant family history.11 Additional detailed phenotyping of the genetic forms of nfvPPA will be required to improve on the continuously evolving understanding of PPA variants within the spectrum of FTLD.
The precise prevalence of nfvPPA is not definitively known because diagnosis requires extensive clinical expertise and available data have largely been derived from tertiary care research center referrals, likely not representative of the general population. However, the 2016 retrospective analysis from the French AD databank identified 24% (23 of 97) of PPA cases with available CSF biomarkers as nfvPPA, with a mean age of disease onset at 60.9 years, an average delay of 2.3 years between first symptom and diagnosis, slight male predominance (52%; 11:12, female/male), and underlying AD pathology in 35% of cases.14 In 2 series of 353 patients with FTLD, 24.6% of cases were identified as nfvPPA.41 An epidemiologic study investigating FTLD syndromes in 2 UK counties with a population of 1.69 million yielded an estimated nfvPPA prevalence of 1.5 per 100,000.42
No pharmacologic option exists to improve or protect against declining function for patients with nfvPPA. However, speech-language treatment has demonstrated efficacy and structured oral reading has been proposed as a treatment method for apraxia of speech in nfvPPA.68 Additionally, tran-scranial direct current stimulation over the left posterior peri-Sylvian region and the Broca area has also been investigated as a potential treatment technique for nfvPPA.69 Supportive care remains the mainstay of PPA treatment, noting that patients with nfvPPA may experience dysphagia and should consult with a dietician or speech therapist for consideration of assisted feeding.11 Early detection of deficits, particularly physical, is critical to optimize outcomes related to changes in functional status. Support groups can also be extremely helpful, both for patients and their caregivers. Patients with nfvPPA who experience limited verbal output but with preserved comprehension may also benefit from alternative forms of communication devices.
Structural Imaging.
The most common imaging feature associated with nfvPPA is regional atrophy predominantly involving the inferior frontal, opercular, and insular regions of the dominant hemisphere (Broadmann area 44/45; Broca area), most commonly on the left, with associated widening of the left Sylvian fissure (Fig 11). Notably, the left inferior frontal gyrus and pars opercularis and triangularis of the left frontal operculum are considered the syndrome-specific epicenters of disease in nfvPPA (Fig 12).70,71 Similar to other PPA variants, while the presence of left, posterior, frontoinsular atrophy is highly suggestive of nfvPPA, its absence does not exclude the diagnosis. A wide variety of nfvPPA imaging patterns has been reported, ranging from no specific pattern of atrophy to left-hemispheric, left-frontotemporal, bifrontal, or even generalized patterns of atrophy, with imaging inconsistencies largely attributable to the clinical and histopathologic heterogeneity of nfvPPA syndromes.72 Given the progressive nature of all PPA subtypes, comparison with prior imaging and serial examinations may reveal subtle region-specific progressive atrophy and should be pursued in the appropriate clinical setting.
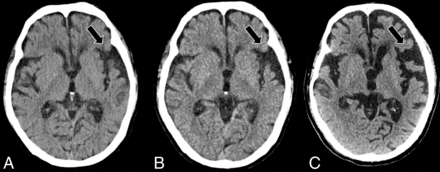
Serial axial CT scans at presentation (A), 2 years post-initial presentation (B), and 4 years post-initial presentation (C) in a right-handed individual with progressive language deficits demonstrate progressive widening of the left-greater-than-right Sylvian fissures (black arrows, A–C), suspicious for nfvPPA.
Axial CT (A), axial T2-weighted MR imaging (B), axial T1-weighted MR imaging (C), coronal CT (D), and coronal T1-weighted MR imaging (E) in a right-handed individual with apraxia of speech demonstrate asymmetric widening of the left Sylvian fissure with predominant left posterior frontoinsular atrophy (black arrows, A–E), suspicious for nfvPPA.
DTI techniques have demonstrated involvement of the dorsal language pathway of long-range WM fibers connecting the frontal, subcortical, and parietal areas, a unique finding in nfvPPA that has not been described in other PPA subtypes,73,74 as well as WM damage in the dorsal pathway (superior longitudinal fasciculus).64 GM atrophy has also been described in the premotor regions, the supplementary motor area, and striatum.10,70 Syntactic processing deficits in patients with nfvPPA have been associated with structural and functional abnormalities involving the posterior part of the inferior frontal gyrus.75 Although most cases affect the left hemisphere, right-hemispheric involvement has also been reported, hypothesized to occur in left-handed individuals or those with a history of developmental learning disabilities, such as dyslexia.76
Longitudinal progression of atrophy in nfvPPA has been reported to involve the posterior frontal regions, supplementary motor area, insula, striatum, inferior parietal regions, and underlying WM.70 Furthermore, atrophy typically progresses from the frontal operculum to the supplementary motor complex through the frontal aslant tract, which plays a role in the initiation and execution of movements, particularly articulation, and ultimately to the basal ganglia and supramarginal gyrus.73 The loss of integrity of the frontal aslant tract in nfvPPA is associated with distortion errors made by patients in spontaneous speech, as well as verbal fluency task performance. Therefore, nfvPPA is an example of a network disorder involving the circuit of regions and connections involved in speech production.77
Functional Imaging.
A resting-state fMRI study demonstrated decreased functional connectivity between the left inferior frontal gyrus and posterior middle temporal gyrus in nfvPPA, even in patients without advanced atrophy.78 Such results suggest the possibility of fMRI serving as a useful imaging technique for the early detection of PPA, particularly nfvPPA, which may ultimately improve patient outcomes as disease-modifying therapies emerge in the clinical setting.
There are limited data regarding the use of ASL for nfvPPA specifically. Further studies assessing the potential role of ASL for patients with nfvPPA as well as its performance compared with [18F] FDG-PET are needed.
Molecular Imaging.
[18F] FDG-PET and SPECT can be performed to demonstrate characteristic asymmetric hypometabolism-hypoperfusion predominantly affecting the left posterior frontal and peri-insular regions, including the left frontal operculum (Fig 13). Specifically, metabolic reduction in the left posterior frontoinsular region, including the inferior frontal gyrus, insula, and premotor and supplementary motor areas, is necessary to make an imaging-supported diagnosis of nfvPPA.6
[18F] FDG-PET cortical surface maps demonstrate an abnormal FDG distribution pattern with severe left-greater-than-right hypometabolism, most pronounced in the dorsal frontal lobes and left peri-insular region (A), with corresponding disproportionate cortical atrophy particularly pronounced in the left insular region visualized on brain MR views (B), findings further supported by semiquantitative FDG-PET analysis using z scores calculated in comparison with age-matched cognitively healthy controls, demonstrating markedly decreased values in the left > right peri-insular region, including in the pars opercularis and pars triangularis of the left inferior frontal gyrus, corresponding to the expected Broca area (B).
Regarding the cortical amyloid burden in nfvPPA, a study investigating [11C]-Pittsburgh compound B in PPA subtypes demonstrated increased binding in only a few subjects with nfvPPA, in an uptake pattern similar to that of AD, including elevated tracer binding throughout the neocortex and striatum.79 A recent study investigating patients with PPA with discordant amyloid status (eg, nfvPPA with AD pathology) found that most cases exhibited FTLD-τ as the primary pathologic diagnosis with AD as an incidental age-related contributon.80 Specifically, 24 of 28 patients (86%) with svPPA and 28 of 31 patients (90%) with nfvPPA had negative amyloid PET findings, whereas 25 of 26 patients (96%) with logopenic PPA had scans with positive findings. The amyloid-positive svPPA and nfvPPA cases with available postmortem data (2 of 4 and 2 of 3, respectively) all had a primary FTLD and secondary AD pathology diagnoses. Therefore, some patients with nfvPPA may have a PET examination positive for amyloid, and the presence of amyloid-positivity is not pathognomonic for any 1 PPA variant.
τ-targeting PET tracers have been used in nfvPPA, most commonly [18F] flortaucipir (AV-1451), a first-generation τ PET ligand, which exhibits increased uptake in the left-greater-than-right frontal operculum and left middle and inferior frontal gyri (Fig 14).19 nfvPPA has also been shown to demonstrate less robust-but-focal uptake in the frontal WM and subcortical structures (Fig 15), regions known to be functionally impaired in nfvPPA, suggesting disease-specific binding to FTLD-4R τ.81 In nfvPPA, AV-1451 has been used to study τ propagation in the left-hemispheric syntactic network, which comprises anterior frontal and posterior temporal nodes connected by the left arcuate fasciculus, with deposition greatest in the 2 nodes of the syntactic network.82 The left arcuate fasciculus also demonstrated decreased fractional anisotropy in nfvPPA, particularly near the anterior node, suggesting τ propagation from node to connected node in human brain networks in the setting of neurodegenerative diseases, including PPA.82
[18F] flortaucipir in nfvPPA. A, On voxelwise comparison with healthy controls, agPPA demonstrates increased uptake in the left-greater-than-right frontal operculum; middle and inferior frontal gyri; and left superior frontal gyrus (pFWE < .05). B, The W score frequency map demonstrates elevated W scores above 1.65 in the bilateral middle frontal gyri and frontal operculum in approximately two-thirds of patients scanned, with voxels above 1.65 in 8 of 11 patients in peak areas. C, ROI analyses reveals group differences in those with in nfvPPA compared with controls in the bilateral pars opercularis (left, P = .0001; right, P = .0018), pars triangularis (left, P = .0016; right, P = .0029), precentral gyrus (left, P = .003; right. P = .0112), and superior frontal gyrus (left, P = .03; right, P = .045). Adapted with permission from Tsai et al.81
Representative [18F] flortaucipir images from 11 patients with nfvPPA and corresponding single-subject W score maps. Tracer retention in the frontal operculum and inferior or middle frontal gyrus is seen in all scans to varying degrees and is more pronounced on the left side. Patients 1–7 show additional bilateral-but-asymmetric frontal WM binding, while patients 8–11 demonstrate mild uptake in the prefrontal cortex. All scans show varying degrees of uptake in the bilateral basal ganglia. Adapted with permission from Tsai et al.81
TSPO-targeting tracers have been explored in PPA, with the goal of characterizing the role of microglial activation and associated neuroinflammation in the pathogenesis of PPA. One study identified significantly increased mean [11C] PK-11195 binding in FTLD (n = 5, including 4 patients with nfvPPA and 1 patient with behavioral-variant frontotemporal dementia) in regions such as the left dorsolateral prefrontal cortex, right hippocampus, and parahippocampus.83
CONCLUSIONS
PPA is a unique and complex spectrum of neurodegenerative disorders that requires a multidisciplinary approach to diagnosis, relying on the aggregate findings of clinical presentation, histopathologic biomarkers, and imaging features. A number of structural, functional, and molecular imaging modalities can support an accurate diagnosis, and neuroradiologists should be familiar with the classic imaging features of each PPA subtype, because prompt and accurate diagnosis may allow improved outcomes and intervention, particularly as disease-modifying therapies enter clinical practice.
Footnotes
This work was supported by the Foundation of the American Society of Neuroradiology Boerger Research Fund for Alzheimer’s Disease and Neurocognitive Disorders (2021): “18F PI-2620 in Primary Progressive Aphasia” (Principal Investigator: Ana Franceschi).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.
- 29.↵
- 30.↵
- 31.↵
- 32.
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- Received October 24, 2021.
- Accepted after revision November 30, 2021.
- © 2022 by American Journal of Neuroradiology