Abstract
BACKGROUND AND PURPOSE: Meningiomas are intracranial tumors that usually carry a benign prognosis. Some meningiomas cause perifocal edema. Resting-state fMRI can be used to assess whole-brain functional connectivity, which can serve as a marker for disease severity. Here, we investigated whether the presence of perifocal edema in preoperative patients with meningiomas leads to impaired functional connectivity and if these changes are associated with cognitive function.
MATERIALS AND METHODS: Patients with suspected meningiomas were prospectively included, and resting-state fMRI scans were obtained. Impairment of functional connectivity was quantified on a whole-brain level using our recently published resting-state fMRI–based marker, called the dysconnectivity index. Using uni- and multivariate regression models, we investigated the association of the dysconnectivity index with edema and tumor volume as well as cognitive test scores.
RESULTS: Twenty-nine patients were included. In a multivariate regression analysis, there was a highly significant association of dysconnectivity index values and edema volume in the total sample and in a subsample of 14 patients with edema, when accounting for potential confounders like age and temporal SNR. There was no statistically significant association with tumor volume. Better neurocognitive performance was strongly associated with lower dysconnectivity index values.
CONCLUSIONS: Resting-state fMRI showed a significant association between impaired functional connectivity and perifocal edema, but not tumor volume, in patients with meningiomas. We demonstrated that better neurocognitive function was associated with less impairment of functional connectivity. This result shows that our resting-state fMRI marker indicates a detrimental influence of peritumoral brain edema on global functional connectivity in patients with meningiomas.
ABBREVIATIONS:
- DCI
- dysconnectivity index
- GLM
- generalized linear models
- MOCA
- Montreal Cognitive Assessment
- PTBE
- peritumoral brain edema
- rsfMRI
- resting-state fMRI
- tSNR
- temporal SNR
Meningioma is the most common intracranial tumor in adults with a reported annual incidence rate of 8.81 per 100,000 inhabitants in the United States.1 The clinical course of patients with meningiomas is usually benign, with long progression-free survival after surgical resection. Most meningiomas are extra-axial tumors, even though some grade 2 meningiomas invade the surrounding brain tissue2 and some authors even suggest that in rare cases, meningiomas can be purely intraparenchymal.3 Even in the absence of brain invasion, healthy brain tissue can be affected by the tumor, illustrated by some meningiomas causing formation of peritumoral brain edema. This suggests that the presence of these tumors, even though extra-axially located, leads to interaction between the tumor and the surrounding brain parenchyma. A number of factors by which meningiomas influence the healthy brain and cause the formation of peritumoral brain edema (PTBE) have been discussed in recent years, including the secretion of vascular endothelial growth factor A, matrix metalloproteinases, and interleukin-6.4 Taken together, there is ample evidence to support an intricate interaction between meningiomas and healthy brain in some cases. This notion is reinforced by the finding that neuropsychological deficits are common in patients with meningiomas. Numerous studies have demonstrated that complex neurocognitive functions like attention, memory, or executive functioning are impaired in patients with meningiomas, indicating that the presence of a meningioma may disturb the complex neuronal networks that are the physiologic basis of these functions.5,6
Recently, functional connectivity measured by resting-state functional MR imaging (rsfMRI) has been suggested as a marker of disease severity in a cohort of patients with gliomas by our group.7 RsfMRI exploits the physiologic fluctuations of oxygenated blood in the brain, which reflects neuronal activity, to assess the functional architecture of the human brain.8,9 The entirety of functional connections in the brain is often referred to as the functional connectome. In our aforementioned study,7 it could be demonstrated that patients with more aggressive gliomas exhibited more impairment of functional connectivity. This outcome established the concept that the individual disease burden of patients with brain tumors can be captured by assessing the impairment of functional connectivity caused by the tumor, using rsfMRI. To this end, we developed an imaging tool that quantifies and visualizes impairment of whole-brain functional connectivity in the individual patient, called the dysconnectivity index (DCI).7 Quantification was based on the deviation of each individual connectivity profile from the distribution of connectivity profiles of a healthy reference group.
In the present study, we intended to extend this concept to patients with suspected meningiomas. Here, it was our goal to assess whether meningiomas affect functional connectivity and whether functional connectivity is influenced by factors like tumor size and the presence of perifocal edema.
MATERIALS AND METHODS
Study Design
Patients with a suspected meningioma on initial contrast-enhanced MR imaging were prospectively included from January 2017 to March 2021. Patients presented with outside MR imaging scans to the skull base clinic at Ludwig-Maximilians-University Hospital. Exclusion criteria were younger than 18 years of age, previous cranial surgery or radiation therapy, and any contraindications to MR imaging such as non-MR imaging–safe metal implants. Patients underwent rsfMRI scanning before craniotomy and resection of the tumors. A histopathologic diagnosis was made according to the criteria set forth in the 2016 version of the World Health Organization grading system.10 Patients underwent neuropsychological testing on the day of the MR imaging procedure using the Montreal Cognitive Assessment (MOCA) test.11 In 2 patients, the MOCA could not be performed because the test was not available in the respective patient’s native language. The study was approved by the local institutional review board and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Scanning Procedure of rsfMRI
RsfMRI was performed as described previously.7 Briefly, acquisition of the MR imaging data on a 3T scanner (Magnetom Skyra; Siemens) included two 6-minute runs with a total scanning time of 12 minutes. Structural MR images were acquired with a sagittal 3D T1WI sequence (radiofrequency pulses and MPRAGE). For details of the MR imaging protocol, please refer to the Online Supplemental Data.
Preprocessing
For all scans, the quality control criteria of the mean section-based temporal signal-to-noise ratio (tSNR) > 100 and mean relative motion <0.5 mm were fulfilled.12 Structural MR imaging data were processed using the FreeSurfer software package, Version 6.0 (http://surfer.nmr.mgh.harvard.edu). For further information please refer to the Online Supplemental Data. Preprocessing of the rsfMRI data sets was performed using FMRIPrep, Version 20.2.2 (https://fmriprep.org/en/stable/) based on Neuroimaging in Python (Nipype https://nipype.readthedocs.io/en/latest/index.html).13 The first 5 functional images were deleted. The remaining frames were standardized and smoothed with a 6.0-mm full width at half maximum Gaussian kernel. MCFLIRT (FSL, Version 5.0.9; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MCFLIRT)14 was performed for the subsequent head motion correction using ICA-AROMA (https://github.com/maartenmennes/ICA-AROMA).15 Last, nuisance regression, filtering, and detrending were performed.15 Nuisance regression included regression of the global signal, CSF, and white matter signal. Filtering comprised high and low band pass filtering, applying cutoff values of 0.01 and 0.08, respectively. For each subject, a correlation matrix of the 3352 voxels for the left hemisphere and 3316 voxels for the right hemisphere was calculated and normalized using z scores.
Calculation of the DCI
The DCI was calculated analogous to methods previously described by Stoecklein et al.7 Also, see the Online Supplemental Data. Briefly, connections that diverge beyond a certain threshold from the distribution of the respective connection in the reference group were counted as “dysconnected.” The individual patient’s dysconnectivity count was then summarized in each hemisphere and normalized to the number of voxels in the respective hemisphere, resulting in the DCI. For each patient, the dysconnectivity count of each voxel was assigned to the respective voxel. The resulting map was smoothed using a Gaussian kernel (full width half maximum = 2 mm) and projected onto each individual patient’s anatomic space.
Shrinking of the Tumor Area
Meningiomas are almost exclusively extra-axial tumors; therefore, that the tumor is embedded in the functional architecture of the brain in a relevant manner is not to be expected. Consequently, we tried to avoid distortion of the functional connectome by minimizing the nonfunctional tumor area in our data set. The Advanced Normalization Tools software package (ANTS http://stnava.github.io/ANTs/) registration is a software tool that uses a mathematic method for performing deformable image registration.16 The algorithm optimizes a deformation field that maps points from one image to the corresponding points in another image, specifically the anatomic atlas being used. When no lesion map is provided to the algorithm, the lesional area is automatically warped and shrunk, resulting in a smaller but potentially imprecisely registered lesion area.
Volumetry
Tumor and edema volume was determined using the Brainlab Elements software package (Brainlab). Tumor volume was semimanually segmented by a board-certified radiologist (S.S.) and a board-certified neurosurgeon (V.M.S.) on the basis of contrast-enhanced T1WIs. Similarly, edema volume was determined by semimanual segmentation of T2WIs. Tumor location was assessed as frontal, temporal, parietal, occipital, or multiple (meaning that the tumor affects >1 lobe).
Statistical Analysis
Generalized linear models (GLMs) were used to conduct univariate and multivariate correlation analyses. Group comparisons were calculated using the Mann-Whitney test. P values ≤ .05 were considered significant.
RESULTS
Patient Characteristics
Twenty-nine patients were prospectively enrolled (mean age, 58.2 [SD, 12.3] years; 22 women). The mean age was higher in patients with PTBE (P = .0014). For details see the Table.
Patient characteristics
Tumor and Edema Characteristics
Twenty-six patients were diagnosed with World Health Organization grade 1 tumors; 3 patients, with grade 2 tumors. The diagnosis of a grade 2 meningioma was made on the basis of elevated mitotic counts in 2 cases and on brain infiltration alone in 1 case. Mean tumor volume for all patients was 24.9 cm3 (range, 2.2–113 cm3). Fifteen patients had no perifocal edema. In patients who had PTBE (n = 14), the mean edema volume was 50.0 cm3 (range, 3.5–118 cm3). Two of 3 patients with grade 2 tumors had PTBE. In these patients, edema and tumor volume were not significantly correlated (r = –0.1101, P = .4903). Tumor volume was higher in patients with PTBE compared with patients without PTBE (P = .0124, see the Table for details).
Impaired Functional Connectivity Is Strongly Correlated with Edema Volume
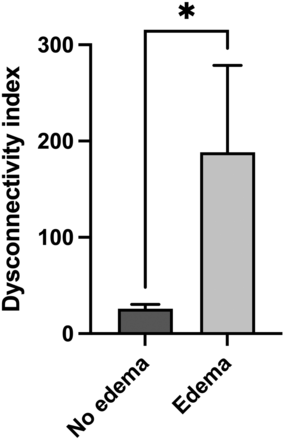
The univariate GLM revealed a highly significant association of edema volume and impaired functional connectivity, as indicated by the DCI (P = .0002). In multivariate GLM analyses adding the potential confounders age, sex, and tSNR, none of these parameters showed a significant association with the DCI (P = .3920, P = .2583, P = .3559, respectively), while the association between edema volume and the DCI remained significant (P = .0023, P = .0002, P = .0011, respectively). Thus, this analysis showed that age did not have a significant influence on the association of PTBE and impaired functional connectivity even though patients with PTBE were older than patients without PTBE. Furthermore, a group comparison between patients without and with PTBE revealed a significantly higher DCI in the group with PTBE (P = .04, Fig 1).
Comparison of the DCI in patients without and with PTBE. The left column shows the mean (SD) in patients without PTBE (n = 15) compared with the mean (SD) in patients with PTBE (right column, n = 14) (*P = .04, Mann-Whitney test).
When restricting the analysis to 14 patients with PTBE, we could grossly replicate our results: Univariate analysis revealed a significant association of edema volume and the DCI (P = .0301). In multivariate GLM analyses adding age and tSNR, neither of these parameters showed a significant association with the DCI (P = .2702 and P = .1718, respectively), while the association between edema volume and the DCI remained significant (P = .0486 and P = .0487, respectively). Only when adding the potential confounder sex did the association between edema volume and the DCI fail to reach significance (P = .0594), while sex itself also did not show a significant association with the DCI (P = .4545). The analysis was furthermore repeated without the patients with grade 2 tumors (n = 26). A significant association of PTBE and the DCI could still be demonstrated (P = .0002).
Abnormal Functional Connectivity Is Not Correlated with Tumor Volume
Tumor volume was not significantly associated with the DCI, neither in a univariate analysis (P = .2345) nor in the multivariate GLM adding age, sex, and tSNR (P = .7771, P = .2094, P = .6843, respectively), even though patients with PTBE were found to have larger tumors. Furthermore, multivariate analyses did not reveal a significant association of tumor volume and tumor location with the DCI (P = .4846 and P = .9228, respectively), while the association between edema volume and the DCI remained significant (P = .0005 and P = .0023, respectively) when adding these parameters to the model. Analogously, in 14 patients with PTBE, when we added tumor volume to the model, the association between edema volume and the DCI remained significant (P = .0309), while no association between tumor volume and the DCI could be detected (P = .4623).
Association of Abnormal Functional Connectivity and Cognitive Performance
Most interesting, we found a significant negative association between abnormal connectivity and the MOCA test results (P = .0023), which stayed significant even after controlling for age, sex, and tSNR (P = .0223, P = .0010, P = .0112, respectively), indicating that a higher DCI is associated with impaired neuropsychological performance. Along these lines, the MOCA showed a significant negative correlation with PTBE (r = –0.5572, P = .0025).
Individual Connectome Maps
Figure 2 shows representative examples of individual connectome maps in patients with meningiomas. Figure 2A displays the connectome map of a patient with a large tumor with extensive PTBE. Here, widespread abnormal connectivity in both hemispheres is demonstrated. Figure 2B depicts the connectome map of a patient with a small meningioma that still causes extensive PTBE. Widespread abnormal connectivity in both hemisphere is also demonstrated. Figure 2C, on the other hand, shows the connectome map of a patient with a large meningioma without PTBE. Here, only very limited abnormal connectivity adjacent to the tumor is observed.
Individual connectome maps in patients with meningiomas. A, Connectome map of a patient with a large meningioma (contrast-enhanced T1WI, first image from the left) with extensive PTBE (T2WI, second image from the left). Connectome analysis shows extensive impairment of functional connectivity in widespread brain regions in both hemispheres (third and fourth images from the left). B, Connectome map of a patient with a small meningioma (contrast-enhanced T1WI, first image from the left), which still causes extensive PTBE (T1WI, second image from the left). Connectome analysis shows extensively impaired connectivity in the whole brain with a focus on regions with PTBE (third and fourth images from the left). C, Connectome map of a patient with a large meningioma (contrast-enhanced T1WI, first image from the left) without PTBE (T2WI, second image from left). Connectome analysis shows minimal disturbance of functional connectivity adjacent to the tumor (third and fourth images from the left).
DISCUSSION
We present a prospective study of patients with newly diagnosed meningiomas. We found that there is a positive association between edema volume and impairment of functional connectivity. We did not find, however, an association between tumor volume and abnormal functional connectivity. We also did not find substantially abnormal functional connectivity in patients without perifocal edema, irrespective of tumor size. Taken together, it appears that substantial interaction between meningioma and healthy brain parenchyma indicated by perifocal edema is necessary for disturbances in connectivity to occur. A possible functional significance for this finding in patients with meningiomas is suggested because abnormal functional connectivity was associated with worse neuropsychological performance as indicated by the MOCA test scores.
These findings could open new avenues of research and possibly lay the foundation for new treatment rationales in patients with meningiomas. We found that the presence of PTBE in patients with meningiomas caused damage to global functional connectivity. This finding might indicate that the biochemical interaction between surrounding healthy brain and tumor, which leads to edema formation, also has functional implications for the whole brain. Taken together this gives rise to the question of how this observed effect of damaged functional connectivity in the whole brain of patients with substantial perifocal edema is mediated. Exactly how PTBE in patients with meningiomas arises is still a matter of intense debate. Many possible causes for edema formation have been discussed in the past; eg, dysfunction of the glymphatic system of the brain has recently been implicated in edema formation.17
It is becoming clearer, however, that increased proinflammatory cytokines play an important role in the formation of perifocal edema. Interleukin-6, which has a prominent role in the regulation of inflammatory processes,18 has been described as a relevant factor in the development of PTBE. It was found that the expression of interleukin-6 was 7-fold higher in tumor cells from meningiomas with perifocal edema.19 Furthermore, a number of other cytokines such as chemokine 12 and transforming growth factor β are being produced in the tumor microenvironment.4 This finding goes to show that meningiomas with PTBE might trigger immunological activity of the host. It is possible that this immunologic activity, in turn, could have a proinflammatory effect in the whole brain.
This change in the inflammatory status of the brain could lead to altered brain function. There is ample evidence to support systemic inflammation altering functional connectivity in the brain. It could be shown, for example, that the infusion of low doses of lipopolysaccharide, which triggers systemic inflammation with a subsequent increase of systemic interleukin-6, led to major changes in functional connectivity as determined by rsfMRI.20 Furthermore, a recent study that investigated the link between systemic inflammation and depression found that functional connectivity was altered in patients with depression, depending on systemic inflammation.21 It appears obvious from these studies that there is an effect of systemic inflammation on brain function that is reflected in altered functional connectivity. A similar effect might occur in our patient cohort because of the local proinflammatory milieu caused by the tumor. This could constitute a pathophysiologic link between PTBE and damaged whole-brain functional connectivity.
Additionally, edema is likely to interfere with local blood circulation and thus with the blood oxygen level–dependent signal per se, possibly accounting for a fraction of the effect that we have observed. Our data suggest that edema affects functional connectivity on a more global level. This suggestion can be substantiated by the fact that disruption of functional connectivity was also observed in the nonlesional hemisphere, as seen in Figs 2A, -B. If there was only a local effect of blood oxygen level–dependent signal distortion, one would expect that disruption of functional connectivity would be limited to the lesional hemisphere.
The observation that whole-brain functional connectivity is altered in patients with meningiomas with substantial PTBE leads to the question of whether this finding has importance for neurocognitive function in these patients. It has been shown in a previous study that the presence of PTBE is associated with worse postoperative cognitive function in patients with meningiomas.22 This finding indicates that PTBE has detrimental effects on neurocognition, even though this study was limited by the small sample size with no preoperative data available. An additional study looking at patients with meningiomas preoperatively, however, also found that PTBE has a detrimental effect on neurocognition when patients with and without edema were compared.23 In summary, there is evidence in the literature that PTBE leads to cognitive impairment that goes beyond what would be expected from mass effect alone. These results support the findings of our study,22,23 in which impaired global functional connectivity was associated with PTBE in patients with meningiomas. Furthermore, impaired functional connectivity was associated, in our study, with decreased neurocognitive performance. We did not, however, find a correlation between tumor size and impaired functional connectivity. This result implies that even very small meningiomas that cause the formation of substantial PTBE also cause impaired functional connectivity with ensuing detrimental effects on neurocognition.
Taken together, our findings suggest that the abnormal functional connectivity associated with PTBE observed in our study could have pathophysiologic significance for the development of neurocognitive impairment in patients with meningiomas. Further prospective studies to test whether functional connectivity improves after resection and consecutive regression of PTBE and whether this improvement is associated with better neurocognitive function seem warranted and have been currently launched at our department.
Limitations
A potential limitation of our study is that our method for measuring functional connectivity in patients with intracranial tumors was originally developed for application in patients with intra-axial tumors. To compensate for this shortcoming, we developed a method to computationally remove the tumors from the imaging space and compute abnormal functional connectivity only in the nontumoral areas of the brain. It cannot be fully excluded that this automated “shrinking” of tumors is a potential cause of errors, eg, by removing nontumoral areas from the imaging space and thus underestimating the degree of damage to functional connectivity. Furthermore, age, sex, tumor volume, and functional data quality (assessed by tSNR) constitute potential confounders. However, in multivariate analyses including these potential confounders, PTBE remained a significant factor for an elevated DCI. Furthermore, we failed to observe a statistically significant association between tumor size as well as age and impairment of functional connectivity, even though patients in the PTBE group were older and had larger tumor volume (Table). Our data thus support the notion that the presence of edema alone, irrespective of tumor size and patient age, is an indicator of pathophysiologic processes, which have an impact on functional connectivity. As a note of caution, we would like to point out that dysconnectivity is calculated in gray matter only but might partially spill into white matter areas in individual dysconnectivity maps due to resolution and smoothing effects.
CONCLUSIONS
We present a prospective study in a cohort of patients with meningiomas with and without perifocal edema. We found that functional connectivity is more impaired in patients with edema, independent of tumor size and other potentially confounding factors. We were also able to demonstrate that less impairment of functional connectivity was associated with better neuropsychological performance. This finding indicates a possible role for our rsfMRI marker as an indicator of pathophysiologic processes with an impact on cognition in patients with meningiomas.
Footnotes
C. Schichor and S. Stoecklein contributed equally to this work.
This work was supported by a grant from Deutsche Krebshilfe (Consortium “Aggressive Meningiomas”) to V.M.S. and C.S.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 3, 2022.
- Accepted after revision May 28, 2023.
- © 2023 by American Journal of Neuroradiology