Abstract
BACKGROUND AND PURPOSE: Variations in the origins and courses of the vertebral arteries are relatively rare but may be clinically meaningful. We hypothesize a relationship between variant origins of the vertebral arteries and their levels of entry to the foramina transversaria.
MATERIALS AND METHODS: In this retrospective study of CT angiograms, we document the frequency and types of vertebral artery variants, correlating origins with levels of entry to the foramina transversaria.
RESULTS: Vertebral artery variants were observed in 18.7% of a sample of 460 CT angiograms of the neck. Right-sided variants were less common than left (44.2% versus 68.6%, with 12.8% bilateral) and more common than previously thought. The most common variant on both sides was a variant origin proximal to the normal vertebral artery origin and entry at C5. Most right vertebral arteries originating within 2 cm of the origin of the right subclavian artery and left vertebral arteries originating between the left common carotid and subclavian arteries were “high-entry” variants. Most “low-entry” variants, entering at C7, took origin from the arch just distal to the left subclavian artery or at a common origin with the costocervical trunk. Multiple origins or accessory vertebral arteries were also described, and each moiety followed the same rules described for single origins. A map of vertebral artery origins mirrored the map of aortic arch embryology.
CONCLUSIONS: Vertebral artery variants follow certain well-defined patterns that correlate with the embryology of the aortic arch and great vessels.
ABBREVIATIONS:
- CCT
- costocervical trunk
- FT
- foramen transversarium
- ISA
- intersegmental artery
- LCCA
- left common carotid artery
- LSCA
- left subclavian artery
- LVA
- left vertebral artery
- RSCA
- right subclavian artery
- RVA
- right vertebral artery
- SCA
- subclavian artery
- VA
- vertebral artery
The vertebral artery (VA) normally takes origin from the subclavian artery (SCA) and enters the foramen transversarium (FT) of the C6 vertebral body. This pattern is found on both sides in most cases, with estimates ranging from 82.7% to 99%.1⇓⇓⇓⇓-6 Variations from this configuration are sufficiently uncommon to warrant case reports on a regular basis,7⇓⇓-10 and several studies have tried to compile these variations through meta-analysis11 or literature review.12 Most larger studies with primary data have described variants either at the site of origin of the VA or the site of entry into the FT. A much smaller number of studies have studied both systematically in concert, and these have noted that left VAs originating directly from the aortic arch may enter the FT at levels other than C6 with greater frequency.1⇓⇓⇓-5,13⇓⇓⇓⇓-18 Of these, 1 study3 noted that those originating distally on the arch enter at C7. Most of these studies found marked variability in right-sided FT entry but little variability in right vertebral artery (RVA) origins,1⇓⇓⇓-5,13⇓⇓⇓⇓-18 a perplexing difference that has not been explained. One notable exception3,7 is that origins on the very proximal right SCA (RSCA) tend to have a higher entry point, but this was not quantified.
More uncommon still are multiple origins of the VAs. These are most frequently described in case reports, though 1 large series found them in only 0.3% of cases.19 Adachi noted, on the basis of his cadaveric series (cited in Bordes et al20), that “accessory” left VAs originate directly from the aortic arch and that accessory right VAs originate more proximally on the SCA. However, the origins and entry points of VAs with multiple origins also have not, to date, been described systematically.
In summary, although a wide range of variations has historically and repeatedly been noted in VA origins and entry points, a systematic approach that could organize and unify these disparate variants has yet to be proposed.
This issue is important because variant courses of the VAs may predispose to intraoperative complications, including injury to an “unprotected” VA that is not within the FT21,22 or a VA whose origin from the aortic arch reduces the space available for placement of a stent.23,24 In addition, direct origin of the left vertebral artery (LVA) from the aortic arch has been associated with an increased risk of dissection,25 making recognition of this variant particularly important.
Here we describe a series of VA variants encountered during the course of normal neuroradiologic duties at an inner-city hospital in the United States (University Hospital, Newark, NJ). We describe their origins and points of entry into the FT and propose a unifying hypothesis linking these patterns with the embryology of the great vessels.
MATERIALS AND METHODS
CTAs of the neck were obtained for a variety of indications, including stroke, trauma, nontraumatic hemorrhage, vertigo, abnormal findings on carotid Doppler ultrasound, and follow-up for findings noted on earlier studies. CTA was performed according to institutional procedures, by using either a 64- or 128-section scanner (LightSpeed VCT or Revolution GSI, respectively; GE Healthcare) with bolus tracking following administration of Omnipaque 350 (GE Healthcare) into the venous system at a rate of 4 mL/s. Slices (0.625-mm-thick) were obtained from below the aortic arch through the circle of Willis or through the entire head, depending on the indication. Coronal and sagittal reformats were obtained, and MIPs were generated. 3D volume-rendered reformats were generated using dedicated software (Syngo Via; Siemens) (Fig 1). The same software was used to generate the images in subsequent images. Measurements of distances along the parent arteries to the origins of the VAs were obtained by generating curved planar reformats and measuring linear distances along the parent vessels (Fig 2). Cases with VA variants were identified and compiled in a deidentified file on a Health Insurance Portability and Accountability Act–compliant encrypted server. This study was exempted from institutional review board review by the Rutgers University Institutional Review Board (Pro 2022000874).
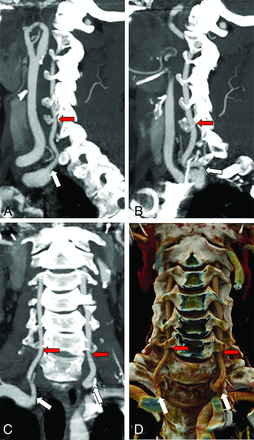
Normal anatomy of the cervical VAs. Sagittal MIPs of the right (A) and left (B) VAs and both in the coronal plane (C), as well as a coronal 3D rendering (D) show the origin of both VAs from the SCAs (white arrows) and their entry into the FT at C6 (red arrows).
Quantification of the distance from the right SCA origin to the origin of the RVA. A, Sagittal MIPs show the origins of the RSCA (yellow arrow), defined as the distal wall of the origin of the right common carotid artery and the RVA (red arrow). B, Curved planar reformat shows a straightened SCA with estimation of the distance to the RVA origin along the SCA (black line indicates distance; yellow line, RSCA origin; red line, RVA origin).
RESULTS
To describe the frequency of variants of VA origin or FT entry in our population, we reviewed an unselected consecutive series of 493 CTAs of the neck, representing all such studies performed during a 3-month period. Of these, 33 were excluded because of patient duplication (a patient scanned more than once in the trial period), an inadequate bolus, excessive motion, or beam-hardening artifacts, making it impossible to ascertain the origin and/or entry point of either VA. In the remaining 460 studies, 86 cases (18.7%) were identified with VA variants. Two-thirds (67.4%) of the variant population presented for a wide range of neurologic conditions, including stroke, intracranial hemorrhage, and surveillance or pre- or postoperative evaluation for treatment of an aneurysm, known dissection, or tumor, while 32.6% presented with trauma. These presentations were comparable with the presenting conditions in the parent population (69.2% for neurologic indications versus 30.2% for trauma). The demographic properties of this subpopulation (59.3% men, mean age, 51.2 years; 40.7% women, mean age, 59 years) were also similar to those of the parent population (55.2% men, mean age, 53.6 years; 44.8% women, mean age, 59.3 years).
Thirty-nine cases demonstrated variants in entry to the FT on the right (8.5% of the total sample and 44.8% of the variant cases, Fig 3), and 59 demonstrated variants in origin and/or entry on the left (12.8% of the total sample and 67.8% of the variant cases, Fig 4). These numbers include 11 with bilateral variants (2.4% of the total sample and 12.8% of the variant cases), so 28 had variants only on the right; 48, only on the left; and 11, bilaterally. These are summarized in Table 1. Three accessory VAs (0.65% of the total sample and 3.5% of the variant cases) were identified in this sample, as well as a single case of triplicated origin (0.22% of the total sample and 1.2% of the variant cases). Considering the frequency of cases with unilateral variants (5.9% on the right and 10.4% on the left), the expected frequency of bilateral variants, assuming independence of the events, was 0.0061, though the observed frequency was 0.024, indicating a 3.9-fold increased likelihood of bilateral variants than would be predicted if the events were independent. We note that in this series, we encountered 6 cases of aberrant right SCAs (1.3%), and 1 right-sided arch (0.20%), within ranges described in the literature.26,27
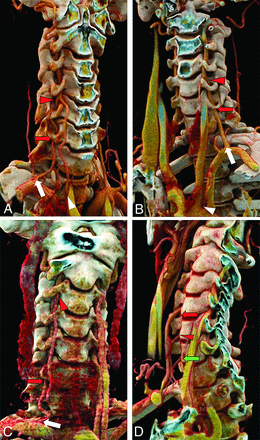
RVA variants. RVA origin within 2 cm of the RSCA origin (white arrows) entering the FT (red arrows) at C3 (A), C4 (B), or C5 (C). D, The RVA originates from the distal aortic arch (white arrow), crosses along the upper thoracic vertebral bodies, and enters the FT at C7 (red arrow). Note that in this case, the left VA also enters the FT at C7 (green arrow), having originated as the fourth branch from the aortic arch (not shown).
Box-and-whisker plot showing the distance from the origin of the RSCA for right VAs entering the FT from C3 through C7. Although there is some overlap, high-entry VAs originate more proximally than arteries entering at C6. There is more heterogeneity in low-entry VAs, which may originate from the aortic arch (negative numbers on this figure) or along the RSCA. For this figure, all low-entry VAs are considered together, without regard for whether they share a common origin with the CCT.
Distribution of variant VA origins and entries to the FT in a consecutive series of 493 casesa
To supplement these observations, we added additional cases of variant VAs that had been accrued in a nonsystematic fashion during the preceding 2 years during the course of routine work in the same hospital. These were added to increase the number of observations of these variants, some of which are extremely rare. Thirty-nine cases demonstrated 1 or both VAs entering the FT at a level other than C6, and 9 angiograms demonstrated accessory vessels for 1 or both VAs (Fig 5). In 3 of these, the contralateral VA had an atypical level of entry (all on the left), yielding a total of 42 single atypical VAs. Of these 42 single VAs, 20 (48%) were on the right side and 30 (71%) were on the left, similar to the relative frequencies in our exhaustive sample. Because this was not an unbiased sample, frequencies relative to the population cannot be calculated.
LVA variants. LVA origin directly from the aortic arch, between the LCCA and LSCA origins (white arrows) entering the FT (red arrows) at C3 (A), C4 (B), or C5 (C). D, The LVA originates as the fourth branch from the arch (white arrow) and enters the FT at C7 (red arrow).
When we reviewed all 125 cases with variant arteries, the most common pattern on the right was origin from the RSCA, with entry to the FT at C6 (Fig 1A, -C, and -D). We found that most (86%) of these typical arteries originated >2 cm from the origin of the RSCA. The most common pattern on the left was the origin from the left subclavian artery (LSCA) near the apex of its curve, with entry to the FT at C6 (Fig 1B–D).
Variants in right VA anatomy are illustrated in Fig 3. The most common variant encountered on the right was the artery originating within 2 cm of the right common carotid artery (30 cases, Fig 3C), with entry to the FT at C5. This was the only variant that demonstrated a statistically significant sex predilection, occurring more commonly in men than in women (21 versus 8, respectively, P = .016). Entry at C3 or C4 was also associated with proximal origin on the RSCA (Fig 3A, -B and Fig 4), and these vessels frequently originated even more proximally than those entering at C5. Entry at C7 was associated with origin either at the distal aortic arch or along the more distal RSCA (Fig 3D).
When the distances of the VA origins on the right were grouped by their FT entry level, there was some overlap, particularly between origins of vessels entering at C5 and C6 (Fig 4). However, there was good overall separation between these groups, and nearly no overlap between “typical” origins and those entering the FT at C3 or C4, which originated more proximally on the RSCA. The origin of arteries entering at C7 from the aortic arch (negative numbers in Fig 4) as well as at more distal locations along the RSCA contributed to a wide range of values for this subset of arteries.
Variants of LVA anatomy are illustrated in Fig 5. The most common variant on the left was direct origin from the aortic arch between the left common carotid artery (LCCA) and the LSCA (39 cases, Fig 5C), with entry to the FT at C5. The next most common variant was origin distal to the LSCA with entry at C7 (Fig 5D). The frequencies of different entries to the FT on both sides are summarized in Table 1.
In the cases with multiple origins (Fig 6), 9 (83%) were on the right, 2 (17%) were on the left, and one (8.3%) was bilateral (Table 2). No sex predilection was found in these cases (7 women and 5 men, P > .05). In the bilateral case, the left side had an accessory artery and the right side had 2 accessory arteries, such that the distal VA had a triple origin (Fig 6D). In all cases of accessory arteries, 1 moiety took origin from the SCA at the usual location for a normal VA, >2 cm from the RSCA origin, and entered the FT at C6. The other moiety took origin either from the RSCA within 2 cm of its origin or directly from the arch between the LCCA and LSCA, joining with the normal VA already in the FT. One aberrant branch on the right entered at C3 (Fig 6C), and one on the left entered at C5. In the remaining cases with accessory arteries, the aberrant moiety entered at C4. In the triplicated case, the C6 origin was close to the normal location along the SCA (17 mm from the RSCA origin), and the other moieties, entering the FT at C4 and C5, originated at 7 and 11 mm, respectively. No accessory moieties entered at C7 on either side.
Multiple origins of the VAs. A, Two moieties originate proximally and distally along the LSCA. The moiety originating more distally (white arrow) at the expected VA origin enters the FT at C6 (red arrow), and the more proximal moiety (white arrowhead), originating within 2 cm of the RSCA, joins the first moiety at C4/C5 (red arrowhead), forming a fused artery that enters the FT at C4. This is the most common multiple-origin pattern. B, A similar pattern is noted on the left with the normal VA origin (white arrow), dominant in this case, entering the FT at C6 (red arrow) and the variant origin, originating from the arch between LCCA and LSCA (white arrowhead), joining at C4/C5 with a fused artery in the FT at C4 (red arrowhead). C, A rare subvariant, with 1 RVA moiety originating 8 mm from the right common carotid artery (not shown) and entering the FT at C3 (red arrowhead) and the other originating normally from the RSCA (white arrow) and entering at C6 (red arrow). D, An extremely rare variant, with the RVA having 3 origins, originating at 7, 11, and 14 mm along the RSCA and entering at C4 (red arrow), C5 (red arrowhead), and C6 (green arrow), respectively.
Distribution of variant VA entries to the FT in an enriched sample of all 125 cases with variant VAsa
When VA variants were mapped by their origins and coded for their level of entry to the FT, these patterns became clearer (Fig 7). While the VAs entering normally at the C6 FT originated from a relatively wide range of distances along the SCA on both sides, virtually all cases of “high-entry” at C3–C5 took origin from relatively narrow vascular segments. Overall, these high-entry vessels (entering FT at C5–C3) originated from a small segment of the RSCA, 93% originating within 2 cm of the RSCA origin. In addition, 2 cases with high-entry originated from the right common carotid artery itself. In contrast, only 14% of VAs entering the C6 FT originated within this range. On the left, these originated from the small segment between the LCCA and the LSCA, or from the proximal segment of the LSCA.
The origins of the VAs plotted against an idealized aortic arch. For this illustration, each VA origin was plotted in a 1D manner as a function of distance from the RSCA origin on the right or from the LSCA on the left. The position in any dimension other than that along the axis of the vessel is not based on anatomic data, and the distribution of vessel origins in the medial-lateral or craniocaudal dimension is used only to permit better visualization of the data points. Typical origins giving rise to VAs entering the FT at C6 are shown in white. Origins of vessels entering the FT at C3, C4, C5, and C7 are shown in purple, cyan, yellow, and red, respectively. Vessels originating along the posterior wall of the LSCA and entering at C7 are shown in red with a white outline. In addition, vessel origins entering at C7 whose vessel origin is shared with the CCT are shown in white with a red outline. Most of the high-entry vessels, which enter at C3–C5, originate in the proximal SCA on the right and directly from the arch or in the proximal subclavian artery on the left. Most of the low-entry vessels originate distal to or along the posterior wall of the LSCA in the region of the aortic isthmus.
Most cases with “low-entry” at C7 originated from the aortic arch distal to the LSCA. A single right VA, originating even more distally from the thoracic aorta, entered the FT at C7 but coursed along the homologous costovertebral joint beginning at T4.
There were exceptions to the patterns we observed. For instance, in 4 cases, the LVA took origin from the aortic arch between the LCCA and LSCA and entered the FT normally at C6. In 5 cases, 2 on the right and 3 on the left, the VA took origin at a normal location on the SCA (defined here as >2 cm from the RSCA origin on the right, as described above) and entered the FT at C7. In 4 of these cases, there was a common origin of the VA and the costocervical trunk (CCT), and in the fifth case (on the left), the VA origin was within a few millimeters of the CCT.
DISCUSSION
The VAs form from longitudinal anastomotic vessels that bridge the cervical intersegmental arteries (ISAs) during development.28 These ISAs supply the somites in the developing embryo. The SCAs derive wholly or in part from the seventh cervical ISAs, and the VAs derive from the anastomotic arteries of the first through sixth cervical ISAs, entering the FT of the sixth vertebral body.29 (For the purposes of this discussion, we use the generally accepted nomenclature, but we acknowledge the dissenting opinion of Padget,30 who considers the SCA as arising from the sixth cervical ISA.) This embryologic origin differs between the right and the left sides (Fig 8A). On the left, the entire SCA derives from the seventh ISA, but on the right, only the more distal portion of the SCA derives from the seventh ISA. However, the great vessels are also derived, in part, from the embryonic pharyngeal arch arteries and other embryonic aortic segments. In the more proximal RSCA, there is a segment derived from the fourth aortic arch and an additional segment derived from the embryonic dorsal aorta.31 The fourth arch segment is also represented on the left as part of the aortic arch between the LCCA and the origin of the LSCA.
A, The embryologic origins of the aortic arch and proximal great vessels, adapted from Schoenwolf et al.31 B, VA origin map (from Fig 7) superimposed on the embryologic map. Note that while most VAs take origin from the seventh ISA segment of the SCA on both sides, the high-entry vessels take origin predominantly from the segments derived from the dorsal aorta and fourth aortic arch, and the low-entry vessels take origin predominantly from the distal aortic arch.
Our data suggest that when the VA enters the FT at a higher-than-normal level, it takes origin from a segment distinct from the 7th ISA, most commonly the pharyngeal fourth aortic arch segment (Fig 8B). This origin explains the similarity in the FT entry point for right-sided vessels taking origin on the very proximal RSCA and for left-sided vessels with direct origin from the aortic arch between the LCCA and LSCA.
Low-entry VAs were a more heterogeneous group. Overall, when either VA entered the FT at a lower-than-normal level (nearly always at C7), it usually originated from the aortic arch segment derived from the left dorsal aorta in the region of the aortic isthmus (the segment between the LSCA origin and the ductus arteriosus; Fig 8B). An unexpected finding was that in 5 of 6 (83%) cases in which the VA had what appeared to be a normal origin on the SCA but entry to the FT at C7, there appeared to be a common origin of the VA and the CCT. In the remaining case (17%), the VA took origin within a few millimeters of the CCT. This feature may point toward yet another variant origin, distinct from the segments described above, and suggests a connection between the CCT and the C7 FT. Among the remaining VAs with origin from the LSCA itself and entering the FT at C7, all had their origins along the posterior wall of the LSCA, which might be conceived of as a domain contiguous to and possibly embryologically inseparable from the typical C7 origin just distal to the LSCA.
Earlier studies have stated that variant origins of the RVA are relatively rare.1,11,32 We found their frequency to be nearly two-thirds that of left-sided variants. This discrepancy may be explained by the failure of most other studies to recognize that the proximal RSCA is fundamentally different from the more distal RSCA. Indeed, 1 earlier study did recognize the unique nature of the very proximal RSCA, and in that study, nearly the same number of right- and left-sided variants were noted.3 We build on these observations by quantitatively defining the atypical origin segment on the RSCA and linking these key findings with the distinct embryologic origin of this segment of the RSCA and its homolog on the left. We found that by using a cutoff of 2 cm from the origin of the RSCA, we captured 93% of high-entry VAs and misclassified only 14% of normal VAs, optimizing our sensitivity for detecting a variant entry. We also note that the variant configuration in which the RVA originates proximally on the RSCA and enters the FT at C5 was the only variant with a statistically significant sex predilection, occurring more commonly in men. We note this finding with some caution because these variants are rare, and the number of cases is small. Further study may bolster or negate this finding, though it does raise interesting questions of the interplay of sex and vascular development.
Although most cases with variant VAs occur on only 1 side, we observed a 3.9-fold increased frequency of bilaterally-variant VAs compared with the frequency that would be expected if the events were truly independent. This observation implies that a variant vessel on one side increases the likelihood of a variant vessel on the contralateral side and suggests that the factor or factors that give rise to these variants may not be restricted only to 1 side but may be influencing other developmental processes, with still undefined effects.
There were, of course, exceptions to the patterns we describe here, and some of these may point toward additional connections not recognized earlier. Among the 5 right VAs with what appears to be a variant origin but a normal entry at C6, the VA took origin between 13 and 19 mm from the RSCA origin. Conversely, in 3 left- and 1 right-sided VA, a normal origin gave rise to an artery entering at C5. Given the wide range of origin sites of normal VAs and the realities of vascular redundancy and tortuosity that are well-described, especially with aging,33 this small number of outliers may reflect this range of values. Finally, on the left, 4 VAs took origin directly from the arch, between the LCCA and LSCA, and entered the FT at C6. We cannot explain these cases at present, but note that they are very uncommon, and future studies may help in understanding their development and significance.
CONCLUSIONS
The numerous variants of the anatomy of the cervical VAs, while relatively rare, are potentially clinically important, both in surgical and endovascular settings. Although they appear to be a diverse group of variants, they tend to follow fairly simple general rules, best understood by considering the embryologic basis of their origins. Overall, these findings suggest a systematic connection between the fourth arch and the higher FT and between the dorsal aorta or CCT and the lower FT. These simple organizing principles can help explain not only the range of variants seen but also parse the otherwise seemingly highly variable course of the RVA, demonstrating that both sides follow the same basic patterns as a function of their embryology.
ACKNOWLEDGMENT
The authors wish to thank Dr Patrick R. Hof for helpful comments and anatomical insights.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received February 1, 2023.
- Accepted after revision June 15, 2023.
- © 2023 by American Journal of Neuroradiology