Abstract
BACKGROUND AND PURPOSE: Potential utility of flat panel CT perfusion imaging (FPCT-PI) performed immediately after mechanical thrombectomy (MT) is unknown. We aimed to assess whether FPCT-PI obtained directly post-MT could provide additional potentially relevant information on tissue reperfusion status.
MATERIALS AND METHODS: This was a single-center analysis of all patients with consecutive acute stroke admitted between June 2019 and March 2021 who underwent MT and postinterventional FPCT-PI (n = 26). A core lab blinded to technical details and clinical data performed TICI grading on postinterventional DSA images and qualitatively assessed reperfusion on time-sensitive FPCT-PI maps. According to agreement between DSA and FPCT-PI, all patients were classified into 4 groups: hypoperfusion findings perfectly matched by location (group 1), hypoperfusion findings mismatched by location (group 2), complete reperfusion on DSA with hypoperfusion on FPCT-PI (group 3), and hypoperfusion on DSA with complete reperfusion on FPCT-PI (group 4).
RESULTS: Detection of hypoperfusion (present/absent) concurred in 21/26 patients. Of these, reperfusion findings showed perfect agreement on location and size in 16 patients (group 1), while in 5 patients there was a mismatch by location (group 2). Of the remaining 5 patients with disagreement regarding the presence or absence of hypoperfusion, 3 were classified into group 3 and 2 into group 4. FPCT-PI findings could have avoided TICI overestimation in all false-positive operator-rated TICI 3 cases (10/26).
CONCLUSIONS: FPCT-PI may provide additional clinically relevant information in a considerable proportion of patients undergoing MT. Hence, FPCT-PI may complement the evaluation of reperfusion efficacy and potentially inform decision-making in the angiography suite.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- eTICI
- expanded Thrombolysis in Cerebral Infarction
- FPCT-PI
- flat panel CT perfusion imaging
- MT
- mechanical thrombectomy
- Tmax
- time to maximum
The current standard for assessing reperfusion success after mechanical thrombectomy (MT) is 2D DSA and it is usually evaluated with the Thrombolysis in Cerebral Infarction (TICI) scale.1 Recognition, treatment, and individualized postinterventional care of residual distal vessel occlusions after MT for proximal large-vessel occlusions are of increasing relevance.2 With the advent of devices dedicated to distal MT and the potential use of intra-arterial thrombolytics after incomplete reperfusion, more treatment options are now available, but none are without risks.3⇓⇓-6 A sensitive and correct assessment of the residual perfusion deficit — at best with an estimation of eloquence — allows for a thorough risk–benefit assessment, which can inform subsequent clinical decisions. However, interventionalists performing the procedure are prone to overestimating the technical success based on standard DSA, biasing the overall estimation of the reperfused territory.7,8 Besides a general overestimation bias, specific locations may also be more prone to false-positive TICI 3 ratings.7,8
Periprocedural perfusion imaging might serve as an adjunct for more accurate evaluation of reperfusion success,9⇓⇓⇓-13 especially in cases of subtle distal occlusions or overlapping capillary phase hypoperfusions, which are hard to detect on DSA runs alone.10⇓⇓-13 Pronounced collaterals with competing flow in peripheral vessels, on the other hand, may result in an underestimation of reperfusion outcome. Postinterventional perfusion imaging might help guide decision-making on whether to pursue adjuvant treatments for patients with incomplete reperfusion.12,13
Presently, there is a paucity of data on feasibility, clinical utility, and diagnostic sensitivity of new-generation flat panel CT perfusion imaging (FPCT-PI) acquired after MT. In this proof-of-concept study, we aimed to qualitatively assess whether FPCT-PI obtained directly in the angiography suite at the end of the intervention could provide additional potentially relevant information on tissue reperfusion status compared with the current standard.
MATERIALS AND METHODS
Patient Population
This is a single-center retrospective observational study. The University Hospital Bern is a tertiary-level care center with a 24 hour stroke service, including availability of 24-hour MT treatment. The University Hospital Bern serves as a referral institution for all patients with acute ischemic stroke (AIS) in central Switzerland covering a population of ∼2 million residents with >2000 patients with stroke treated annually. All consecutive patients with AIS admitted between June 2019 and March 2021 who underwent MT were screened for eligibility. Patients with internal carotid artery or middle cerebral artery (M1 or M2 segment) occlusion who had immediate postinterventional FPCT-PI were considered for the final analysis, as these occlusion patterns usually show highest interrater agreement with regard to grading of reperfusion success. Patient records and clinical charts were screened for any adverse events related to additional radiation or contrast exposure during the hospital stay. This study was approved by the local ethics committee (reference ID 231/14, 2019-00547, 2023-00892) and performed according to the standards of the Declaration of Helsinki. Study data are available from the corresponding author upon presentation of a research plan and clearance by the ethics committee. Reporting was performed according to the Strengthening the Reporting of Observational Studies in Epidemiology statement.14
Flat Panel CT Imaging Acquisition and Perfusion Imaging Postprocessing
Details on FPCT-PI image acquisition have been previously reported.15 In short, we used a biplane flat panel CT (Artis Icono and Artis Q; Siemens) with 10 rotational sweeps (5 seconds per sweep with 1-second turnaround) of the C-arm system with Z-axis covering the entire brain. Perfusion maps were computed by using an offline prototype software provided by Siemens Healthineers.16,17 Perfusion maps were computed by using deconvolution-based perfusion analysis.18 Present software demonstrated almost equivalent results to syngio.via (Siemens) when using time to maximum (Tmax) and TTP maps (Pearson correlation coefficient 0.95–0.98 for qualitative analysis).17,19 Further details on FPCT-PI acquisition and perfusion postprocessing are available in the Online Supplemental Data.
Rating on DSA and FPCT-PI
Study ratings for all imaging data were performed independently of the intervention by a core lab (years of neuroradiology training: >10, >4, and >3 years) blinded to technical details and clinical data. The interrater agreement is reported with Krippendorff alpha coefficient. Reperfusion grading was performed on the final anteroposterior and lateral whole-brain DSA runs, which were acquired immediately before the FPCT-PI. Degree of reperfusion was graded by using the expanded TICI (eTICI) scale on the final DSA series.1 Locations of the residual occlusions after MT were classified into frontal, parietal, temporal, and occipital regions (Online Supplemental Data).20 For comparative purposes, we also reported TICI reperfusion as graded by the operating interventionalist at the end of the procedure, by extracting the scores from the acute interventional report that was filled out immediately after the procedure. Whenever the eTICI is reported, it refers to the core lab adjudicated grading, whereas TICI refers to the operators’ assessment, as no eTICI was available from the operators’ reports.
Presence or absence of hypoperfusion findings was rated on postprocessed perfusion maps, which were acquired immediately after the intervention. Presence of hypoperfusion was defined as focal, wedge-shaped perfusion delay within the initial target territory, suggesting a residual vessel occlusion. Careful correlations with preadmission imaging were done, so that perfusion abnormalities related to chronic parenchyma deficits or cervical vessel stenosis were not considered as remaining vessel occlusions. Tmax and TTP perfusion maps were used for qualitative visual evaluation of persisting perfusion deficits as per the definition stated above. Tmax and TTP have the highest sensitivity for early detection of perfusion abnormalities caused by vessel occlusions and show the best correlation to the perfusion maps generated by commercially available software.10,17,19 Classification of the location of the hypoperfusion on FPCT-PI was done by using the same regions as for the DSA maps (Online Supplemental Data). The Core lab was blinded to DSA findings for FPCT-PI evaluation and vice versa. For all patients with hypoperfusion on FPCT-PI, manual segmentation of the hypoperfused area was performed with 3D Slicer (http://www.slicer.org) to obtain hypoperfused tissue volumes (Online Supplemental Data and respective caption for Methodology).21 Hypoperfusion on FPCT-PI was correlated with follow-up imaging at 24 hours regarding the evolution of new infarcts in the residually hypoperfused territory on FPCT-PI, ie, a new infarct occur in the area of hypoperfusion on FPCT-PI that was not evident on admission imaging (Online Supplemental Data and respective caption for Methodology).
DSA and FPCT-PI Agreement
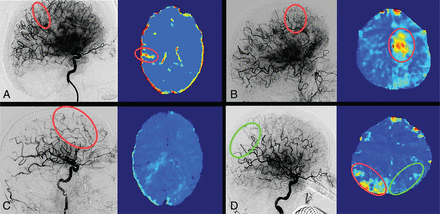
To determine the agreement between the findings on final DSA runs and FPCT-PI, all patients were allocated to 1 of 4 groups. Groups 1 and 2 included patients with concurring dichotomized classification of presence or absence of hypoperfusion on both imaging modalities. For this purpose, patients were classified into no evidence of hypoperfusion on DSA (ie, eTICI 3) or evidence of hypoperfusion on DSA (eTICI <3). Likewise, FPCT-PI findings were dichotomized into presence or absence of hypoperfused areas. Further specifications were made accordingly. In group 1, hypoperfusion findings were perfectly matched by location, for example, TICI 2b hypoperfusion in the parietal area on DSA matched with hypoperfusion deficit in the same parietal area on FPCT-PI (Fig 1A, -B). In contrast, group 2 included patients with concurring dichotomized hypoperfusion findings but a mismatch in the locations of the hypoperfused areas between the two imaging modalities, for example, TICI 2b patient without reperfusion in the parietal area on the DSA but with hypoperfusion in both the parietal and temporal regions on the FPCT-PI (Fig 1C). Groups 3 and 4 included patients with discrepant dichotomized classification of presence or absence of hypoperfusion between the two imaging modalities. In group 3, this discrepancy was defined as complete reperfusion on the final DSA (ie, TICI 3) but clearly demarcated hypoperfusion on FPCT-PI (Fig 2A, -B). Group 4 patients had incomplete reperfusion on the final DSA run (TICI <3) but no hypoperfusion findings on FPCT-PI (Fig 2C). Results from groups 2, 3, and 4 were categorized as potentially clinically relevant, as dichotomized hypoperfusion findings in these 3 groups were mismatched and might reveal additional details on tissue reperfusion status. Core lab graded eTICI scores were used in the initial analysis of agreement between DSA and flat panel CT and for group allocation. To assess sensitivity, we also performed a subanalysis between DSA and FPCT-PI agreement based on operator-graded TICI scores.
Concurring ratings regarding the presence of hypoperfusion. A, Patient with a left-M1 occlusion with complete reperfusion (eTICI 3) on the final angiography imaging and normal perfused tissue on FPCT-PI. B, Patient with a left-M1 occlusion with incomplete reperfusion (eTICI 2b50) on the final angiography imaging and corresponding frontal and parietal hypoperfusion deficits on the FPCT-PI. C, Patient with a left-M1 occlusion with incomplete reperfusion (eTICI 2b67) on the final angiography series, with a clear deficit in the parietal region. On follow-up FPCT-PI, the corresponding hypoperfusion is visible in the same area (green circle), with an additional hypoperfusion noted in the temporal region (red circle). The temporal branch occlusion was not noted by the core lab, but confirmed after making FPCT-PI available. D, Patient with a right-M2 occlusion with incomplete reperfusion on the final angiography series (eTICI 2b67, green circle) and hypoperfusion on the contralateral unaffected side (red circle).
Discrepant ratings regarding the presence of hypoperfusion. A, Patient with a right-M1 occlusion rated as complete reperfusion (eTICI 3) on the final angiography imaging, but follow-up FPCT-PI revealed distal nonperfused tissue. A small residual M4 branch occlusion was confirmed by the core lab after making FPCT-PI available. B, Patient with a left-ICA occlusion rated as complete reperfusion (eTICI 3) on final angiography imaging, but follow-up FPCT-PI revealed a new hypoperfusion due to a distal frontal anterior cerebral artery branch occlusion. C, Patient with a left-M1 occlusion with incomplete reperfusion (eTICI 2b67) on the final angiography series with a clear capillary phase deficit in the frontal region. Follow-up FPCT-PI showed complete delayed reperfusion without any perfusion delays. D, Patient with a left-M1 occlusion with a small persisting deficit in the parietal region on the final angiography run (eTICI 2c, green circle). Follow-up FPCT-PI shows no perfusion delay on the left side; however, there was a hypoperfusion deficit on the contralateral side (red circle).
RESULTS
During the study period, 251 patients with AIS were treated with MT. One of 5 neurointerventionalists at our center acquired postinterventional FPCT-PI during the study period. Fifty AIS cases were treated by this operator, and the decision to acquire FPCT-PI was with the operator, usually dependent on working hours versus off hours and the availability of a technician trained in acquiring FPCT-PI. During the initial study period, perfusion postprocessing failed in 4 patients due to failed timing of contrast bolus and the start of image acquisition. The remaining 26 patients were included in the agreement analysis (Fig 3). In the final study cohort, 69% of the patients were male; mean age was 77 years (IQR, 61–83); and median NIHSS score at admission was 15 (IQR, 11–20). Medium DSA-to-FPCT-PI time was 4 minutes 18 seconds (IQR 3 minutes 12 seconds–7 minutes 30 seconds). Median dose of contrast agent used for angiography and FPCT-PI was 200 mL (IQR, 183–228 mL). Hence, the median additional contrast dose required for FPCT-PI constituted 30% of the total contrast agent dose. Other baseline, interventional, and outcome characteristics are presented in Table and Online Supplemental Data. There were no differences between patients with and without FPCT-PI during the study period (Online Supplemental Data). Interrater agreement was very good (for DSA: 0.87, 95% CI, 0.74–0.94; for FPCT-PI: 0.88, 95% CI 0.82–0.94). Compared with the final DSA imaging, postinterventional perfusion imaging revealed new and potentially clinically relevant information in more than one-third of patients (10/26).
Study flow chart and patient allocation based on the agreement between the findings on final DSA runs and FPCT-PI.
Baseline and Interventional Characteristics
Concurring Ratings Regarding the Presence of Hypoperfusion
The ratings of presence or absence of any hypoperfusion were concurring in 21/26 patients, and these constituted groups 1 and 2. In group 1 (16/26), hypoperfusion findings were perfectly matched by location and size between the 2 imaging modalities. Three patients had complete reperfusion (eTICI 3) with corresponding findings on the FPCT-PI (ie, no hypoperfusion noted; Fig 1A). Thirteen patients had incomplete reperfusion on the final DSA run (eTICI <3), which precisely matched the areas and size of hypoperfusion on the FPCT-PI (Fig 1B). In group 2 (5/26), there were findings of hypoperfusion location mismatch, where on both imaging modalities there was a clear case of demarcated hypoperfusion, but the location and regions of hypoperfusions were mismatched. In all 5 patients, the core lab found more hypoperfused area on FPCT-PI than on DSA (Fig 1C).
Discrepant Ratings Regarding the Presence of Hypoperfusion
Disagreements regarding the presence or absence of hypoperfusion were found in 5/26 patients encompassing groups 3 and 4. DSA-to-FPCT-PI time did not differ between the 5 patients with discrepant dichotomized hypoperfusion ratings and those with concordant dichotomized hypoperfusion ratings (4 minutes 12 seconds versus 4 minutes 18 seconds, P = .9). In group 3 (3/26), reperfusion was rated as complete (eTICI 3) on DSA, but there were hypoperfusion findings on the FPCT-PI. After making FPCT-PI for 2 group 3 patients available to the core lab, reassessment of the final DSA showed peripheral occlusions corresponding to the hypoperfusion findings on FPCT-PI (Fig 2A, -B). In group 4, 2 patients had incomplete reperfusion on the DSA run, but no hypoperfusion on FPCT-PI (Fig 2C). Upon reevaluation of DSA images, the core lab did not change their rating. Individual median DSA-to-FPCT-PI time for these 2 particular cases was 2 minutes 14 seconds and 10 minutes 31 seconds.
A sensitivity analysis for concurring and discrepant ratings regarding the presence or absence of hypoperfusion based on operator-graded TICI scores showed a higher number of potentially clinically relevant cases (13/26). The highest increase was seen in patients allocated to group 3, as operators had a tendency to rate more cases as complete reperfusion when using DSA alone (operator versus core lab adjudicated group 3 rates: 31% versus 11%, Online Supplemental Data). Contralateral to the affected hemisphere with the MT target territory, we noted hypoperfusion findings in 3 patients (Figs 1D-,2D; Online Supplemental Data and respective caption for Results).
Perfusion Imaging and Clinical Outcomes
In total, operators graded reperfusion as complete on the final DSA (TICI 3) in 16/26 patients. Operator overestimation of final reperfusion score occurred in 10/26 patients compared with the core lab ratings on DSA (Online Supplemental Data), and all of these patients showed hypoperfusion on FPCT-PI. There were new infarcts on follow-up within area of FPCT-PI hypoperfusion in 10/26 cases, 5 of these belonging to group 1 and the other 5 to group 2.
Subanalyses on different patient subgroups (stratified by TICI, eTICI, presence or volume of perfusion deficit) indicated better clinical outcome for patients with higher reperfusion scores, without perfusion deficits on FPCT-PI and with lower hypoperfusion volumes (Online Supplemental Data). Two patients had a marked increase in creatinine levels during the acute hospital stay (Online Supplemental Data), but only 1 of them fulfilled the diagnostic criteria for acute kidney injury.22 In these 2 patients, acquisition of FPCT-PI constituted 30% and 37% of the total contrast media dose, respectively.
DISCUSSION
This proof-of-concept study demonstrates that FPCT-PI provides additional and potentially clinically relevant information on tissue reperfusion status for more than one-third of patients undergoing immediate perfusion imaging after thrombectomy. In a considerable percentage of patients, interpreting final reperfusion status based on DSA alone will underestimate the total area of hypoperfused territory. Underestimation is even greater when considering assessments by the operators during the acute treatment phase. Other discrepant findings between DSA and FPCT-PI observed in this study may relate to persistent microvascular obstruction or early spontaneous reperfusion, findings that deserve further studies.
Value of Perfusion Imaging
The additional value of immediate perfusion imaging after MT is unknown. Several studies performed perfusion imaging after MT, but timing of the follow-up images varied.12,23,24 When using immediate perfusion imaging, we observed more sensitive delineation of hypoperfusion with additional territory involvement than with DSA alone. In 5/26 patients with matching dichotomized hypoperfusion grading, we observed additional territories of hypoperfused tissue on FPCT-PI. Interpretation of hypoperfused areas on gray-scale DSA images without spatial resolution can be complex, in particular when performed under time restraints or off-hours,25 especially because most interventionalists tend to overestimate the percentage of reperfused tissue in the acute setting.7 Notably, operators overestimated TICI reperfusion in a considerable number of cases; and in all cases, false TICI readings would have been revealed if perfusion maps had been available during or directly after the acute treatment. In this study, the decision on pursuing additional treatment was made by the interventionalist; processed FPCT-PI was not available at the time point of decision-making, only 4D angiography, which is automatically calculated from the acquisition. FPCT-PI was obtained with a prototype software package offline, and the software cannot yet be used for real-time decision-making.
Several studies have previously shown good correlation between perfusion maps on standardized CT and MR imaging protocols and FPCT-PI,26,27 including a multicenter analysis.28 The overall quality of FPCT-PI maps in this study was sufficient, and the percentage of maps that failed perfusion postprocessing on the FPCT-PI was comparable to the rates of failed postprocessing on standardized CT and MR imaging perfusion protocols.29 Specifically, in all 4 patients with failed postprocessing, the failure was due to timing imbalances of contrast application and start of FPCT-PI rotational runs, a systematic error that occurred during the early study period.
Spontaneous Reperfusion
In 2 patients, there were no hypoperfusion findings on FPCT-PI, although they clearly had incomplete reperfusion (eTICI <3) on the final DSA run. Spontaneous reperfusion after incomplete reperfusion is known to occur frequently.10,12,23 Previous analysis on spontaneous reperfusion reported rates of up to 60% of patients when evaluating 24 ± 12-hour follow-up perfusion imaging.10,23 Even when perfusion imaging is performed 30 minutes after the intervention, it revealed spontaneous reperfusion in almost one-half the patients with a final reperfusion grade of mTICI ≥ 2b.12 Excellent pial collaterals could have masked residual distal occlusion; however, in our 2 patients, we saw no rapid collateral blood flow to the ischemic territory on the final DSA runs. Potential explanation for spontaneous reperfusion could be that FPCT-PI was not thresholded to properly identify residual hypoperfusion. It could have also been an artifact finding, especially as in 1 of these 2 cases there was hypoperfusion on the contralateral, unaffected, side. In the other case, a longer DSA-to-FPCT-PI time (median time 10 minutes 30 seconds) could have increased the chances of delayed reperfusion on FPCT-PI.
New infarct on follow-up imaging was seen in 5/16 patients from group 1 and all patients from group 2. Five patients from group 1 who developed a new infarct in the area of FPCT-PI hypoperfusion generally had lower final reperfusion scores and slower collateral flow, which could explain the occurrence of new infarcts.10,30 On the other hand, all patients in group 2 had additional perfusion deficit that was difficult to detect on DSA alone and could have been potentially treated if FPCT-PI were immediately available.
Persistent Microvascular Hypoperfusion
Immediate postinterventional FPCT-PI could enable the evaluation of microvascular reperfusion and with it the discrepancy between microvascular and macrovascular reperfusion (ie, detection of the no-reflow phenomenon). Reports on detection of microvascular reperfusion on follow-up perfusion imaging are heterogeneous.12,23,24 Even though tissue reperfusion is time-dependent,31 these discrepant findings between the studies are probably related to the lack of standardized criteria for defining microvascular hypoperfusion.32 We found 1 patient (group 3) with potential persistent microvascular hypoperfusion without any distal vessel occlusions on the retrospective DSA analysis (Online Supplemental Data). However, we would be reluctant to classify this as a no-reflow phenomenon because of uncertainties regarding its definition. Use of different perfusion imaging modalities, quantitative cutoffs, and follow-up times are the most likely reasons for discrepant definitions and rates of the no-reflow phenomenon across studies.32
Impact of FPCT-PI on the Workflow
One advantage of FPCT-PI is that it offers a simple workflow. With this technique, it is possible to get information about the patients’ perfusion and potentially tissue status, while options for intra-arterial treatment are still available. Currently, FPCT-PI is not available for all angiography suites, and commercial software packages are lacking. With more advanced tools and streamlined workflows in the angiography suite, FPCT-PI may be a real-time evaluation tool for decision-making in the future.33 Standardized training of nurses and interventionalists on the use of FPCT-PI is required; however, having a clear set of indications and scenarios for FPCT-PI could streamline its implementation in the operating protocol.33 Other potential strategies to mitigate misinterpretations from the DSA could be with the use of validated deep learning-based approaches, especially in suspicion of distal thromboembolism.34 Automating and quantifying perfusion deficits on DSA could minimize the risk of interobserver and intraobserver variability (eg, experience of the rater, inspection attentiveness, TICI scale variations).35 Moreover, combination of standard DSA with perfusion imaging (ie, DSA perfusion) could be useful in quantifying the degree and nature of reperfusion after the intervention.33
Potential Risks of FPCT-PI
FPCT-PI is not devoid of misinterpretations and artifacts.36 Notably, postprocessing failed in 4/30 cases. However, deconvolution-based parametric maps could offer higher sensitivity to alteration in hemodynamics and higher quantitative reliability of perfusion deficits compared with DSA maps.36 Moreover, if the interventionalist observes a perfusion deficit of FPCT-PI, it might prompt them to reevaluate the DSA for residual occlusion. Other potential risks of FPCT-PI could be related to the increased contrast agent and radiation doses.37 However, there does not seem to be any difference between effective radiation dose from FPCT-PI and standard multidetector CT protocols (2.88 mSv versus 2.17 mSv) when using the current FPCT-PI protocols with collimation.38 If FPCT-PI were to become standard of care, the theoretical minimal stochastic risks from such overexposure to radiation in a large pool of patients would have to be balanced by — yet to be shown — individual benefits of therapeutic approach modifications derived from FPCT-PI. Concerns have also been raised about the impact of administering additional contrast agent. In the present cohort, 2 patients had increased creatinine levels; however, it is difficult to determine whether this change is driven purely by additional contrast agent administered for FPCT-PI, especially considering that a more complex MT procedure can easily result in a comparable dose of total contrast. Overall, data consistently show a low risk of contrast-induced acute kidney injury after MT, even in patients with chronic kidney disease.39⇓-41 General consensus is that the benefits of detecting and evaluating eloquence of remaining residual occlusions are likely to outweigh the minimal risk of contrast medium–associated kidney failure.39⇓-41
Limitations
This is a single-center retrospective proof-of-concept study performed in highly selected patients, which prompts confounding and selection bias. Even though Tmax and TTP maps are most sensitive to perfusion abnormalities,10 they might not be sensitive enough to detect hypoperfusion on a microvascular level; other perfusion maps should be considered for a more comprehensive assessment of the microvasculature. Patient clinical outcome was not the focus of the present manuscript; because of the limited sample size, we advise caution when interpreting these results. Despite our best efforts to mitigate possible signal-induced errors in perfusion maps, there could still be effects that are masked or unaccounted for. Specifically, there were fewer runs with FPCT-PI resulting in worse time-resolution than conventional CT perfusion imaging. Moreover, previous intra-arterial contrast injection and accumulation of that contrast in territories of brain-barrier breakdown could influence the calculation of attenuation-time curves.
CONCLUSIONS
Immediate postinterventional FPCT-PI may provide additional clinically relevant information in a considerable percentage of patients undergoing MT. Potential benefits of FPCT-PI may be its high sensitivity for residual vessel occlusions, taking into account collaterals from different territories and determining anatomic location of hypoperfused areas. If these findings are confirmed, FPCT-PI may be used to complement the evaluation of reperfusion efficacy following acute stroke interventions in the future.
Footnotes
A. Mujanovic and C.C. Kurmann contributed equally to this work.
P. Mordasini and J. Kaesmacher contributed equally to this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received September 12, 2023.
- Accepted after revision November 14, 2023.
- © 2024 by American Journal of Neuroradiology