Abstract
BACKGROUND AND PURPOSE: The use of MR imaging in emergency settings has been limited by availability, long scan times, and sensitivity to motion. This study assessed the diagnostic performance of an ultrafast brain MR imaging protocol for evaluation of acute intracranial pathology in the emergency department and inpatient settings.
MATERIALS AND METHODS: Sixty-six adult patients who underwent brain MR imaging in the emergency department and inpatient settings were included in the study. All patients underwent both the reference and the ultrafast brain MR protocols. Both brain MR imaging protocols consisted of T1-weighted, T2/T2*-weighted, FLAIR, and DWI sequences. The ultrafast MR images were reconstructed by using a machine-learning assisted framework. All images were reviewed by 2 blinded neuroradiologists.
RESULTS: The average acquisition time was 2.1 minutes for the ultrafast brain MR protocol and 10 minutes for the reference brain MR protocol. There was 98.5% agreement on the main clinical diagnosis between the 2 protocols. In head-to-head comparison, the reference protocol was preferred in terms of image noise and geometric distortion (P < .05 for both). The ultrafast ms-EPI protocol was preferred over the reference protocol in terms of reduced motion artifacts (P < .01). Overall diagnostic quality was not significantly different between the 2 protocols (P > .05).
CONCLUSIONS: The ultrafast brain MR imaging protocol provides high accuracy for evaluating acute pathology while only requiring a fraction of the scan time. Although there was greater image noise and geometric distortion on the ultrafast brain MR protocol images, there was significant reduction in motion artifacts with similar overall diagnostic quality between the 2 protocols.
ABBREVIATIONS:
- EPIMix
- multicontrast echo-planar imaging
- ms-EPI
- multishot echo-planar imaging
- ss-EPI
- single-shot echo-planar imaging
MR imaging offers exquisite sensitivity and accurate characterization of many acute neurologic conditions, including stroke, traumatic brain injury, and intracranial infection. Utilization of MR imaging as the initial imaging technique in the emergency and inpatient settings, however, has been limited because of long scan time and image quality degradation by patient motion. Lack of timely access to MR imaging for acute ischemic stroke and for hospitalized patients, in general, has been shown to be associated with longer length of hospitalization.1⇓⇓-4 Delays in diagnosis and management, as well as longer length of hospitalization, contribute to greater institutional cost burden.5 Therefore, there is a growing need to increase accessibility to emergent MR imaging use to improve the diagnosis and management of a wide variety of acute intracranial pathologies.6⇓-8
Long scan times associated with MR imaging result in high sensitivity to motion, a particular challenge in emergency department and inpatient settings. Andre et al5 found that there was a 4-fold increase in the incidence of moderate to severe motion artifacts for inpatient and emergency department examinations (29.4%) compared with outpatient examinations (7.5%).5
Techniques to improve the efficiency of clinical brain MR examinations in acute settings have gained increasing attention. Approaches by using 2D TSE sequences and conventional parallel imaging have been previously validated but suffer from substantial signal-noise-ratio losses when pushed to higher acceleration factors, limiting the acceleration that can be achieved.9,10 Alternatively, single-shot echo-planar imaging (ss-EPI) sequences, which acquire all k-space data in a single excitation, have been recently proposed to achieve more dramatic acceleration.11,12 However, ss-EPI suffers from artifacts, including geometric distortion, signal drop-out, and T2* mediated blurring, limiting diagnostic quality, and clinical utility.
Multishot echo-planar imaging (ms-EPI) is a highly efficient interleaved EPI technique that utilizes multiple excitations to reduce echo-train length.13 The increased pixel bandwidth along the phase encoding direction, which comes along with segmented k-space acquisition, reduces off-resonance effects, such as geometric distortion, susceptibility artifact, and signal drop-out.13⇓⇓-16 In addition, artificial intelligence–assisted reconstruction has achieved reconstruction times that are clinically negligible and are able to further reduce noise and aliasing in accelerated MR imaging techniques. We applied the ms-EPI technique to develop a 2-minute ultrafast brain MR imaging protocol consisting of ms-EPI accelerated T1-weighted, T2/T2*-weighted, FLAIR, and DWI sequences. The accelerated images are further denoised by using artificial intelligence–assisted reconstruction. This study investigated the clinical feasibility and diagnostic performance of the 2-minute ultrafast brain MR imaging protocol in the emergency department and inpatient settings.
MATERIALS AND METHODS
This prospective comparative study was performed at a single institution (Massachusetts General Hospital), and was approved by the Institutional Review Board and was compliant with the Health Insurance Portability and Accountability Act. The Institutional Review Board waived the need for signed informed consent.
Study Population and MR Protocol
Consecutive adult patients >18 years of age who underwent clinical brain MR imaging with the “routine brain without contrast” MR imaging protocol in the emergency and inpatient settings were included in the study. All patients underwent brain MR imaging with the 10-minute reference brain MR imaging protocol first (parameters are listed in Online Supplemental Data), followed by the ultrafast 2-minute brain MR imaging examination (parameters are listed in Online Supplemental Data). The 2-minute ultrafast brain MR imaging protocol was implemented by using an ms-EPI research pulse sequence and reconstruction algorithm. Details of the ms-EPI acquisition parameters and the 2-minute ultrafast brain MR imaging protocol are described by Clifford et al.17 The 2-minute ultrafast brain MR imaging protocol consisted of 5 sequences: sagittal T1-weighted imaging, axial T2-weighted imaging, axial T2*-weighted imaging, axial FLAIR, and axial DWI (including ADC reconstruction from the DWIs). Data for the T2 and T2*-weighted images were obtained from the same spin-echo scan via the incorporation of an additional free induction decay (FID) readout before the refocusing pulse (for more details, see Clifford et al,17 their supplemental data). The reference routine brain MR imaging protocol consisted of the same 5 sequences with standard parameters used at our institution. All brain MR examinations were performed on one of two 3T scanners at our institution (Magnetom Prisma and Magnetom Skyra, Siemens Healthineers), by using either a 20-channel head-neck coil or a 32-channel head-only coil. Demographics of the study subjects and clinical indications for MR imaging are listed in Online Supplemental Data.
Machine-Learning Assisted Reconstruction
The raw k-space data from the ultrafast brain MR protocol were extracted from the scanners within 48 hours of acquisition and retrospectively reconstructed by using machine-learning assisted reconstruction. The machine-learning reconstruction algorithm mitigated aliasing and g-factor noise amplification to improve the SNR of the highly accelerated ms-EPI. A detailed description of the reconstruction method was previously described by Clifford et al.17 Briefly, the training data for the neural network were previously collected on a 3T system (Magnetom Prisma) from 16 healthy subjects (8 men, 8 women, ages 19–67 years). The machine-learning–based reconstruction incorporated a tunable parameter for controlling the level of denoising and has been previously validated across various acceleration factors, contrasts, and SNR conditions.
Clinical Evaluation of the Optimized 2-Minute Brain MR Imaging Protocol
All images were reviewed independently by 2 neuroradiologists (O.R. and B.P.A., with 21 and 6 years of experience, respectively). The reviewing neuroradiologists were blinded to the clinical history and image acquisition method. The main clinical diagnosis was scored as a categorical variable with options of “no acute findings,” “recent infarct,” “intracranial hemorrhage,” “mass,” or “hydrocephalus.” For all cases, the ms-EPI was reviewed first, without seeing the reference sequences, and the neuroradiologists were asked to provide the main clinical diagnosis. The reference sequence images were then subsequently reviewed and the neuroradiologists were asked if there was a change in final diagnosis after reviewing the reference sequences.
Overall image quality was evaluated in a blinded head-to-head comparison between the 2 protocols. The screen position (left versus right) of the ms-EPI sequence images and the counterpart reference images, as well as the order of the cases were all randomized. All cases were graded on a 5-point Likert scale, where positive numbers favored the images on the right side of the screen and negative number favored the images on the left side of the screen. The following image quality variables were evaluated by using this scale: image noise, motion artifact, geometric distortion, other artifacts, and overall diagnostic quality. Disagreements between readers were adjudicated by a third neuroradiologist (J.C.).
Statistical Analysis
Comparative image quality analysis between the ultrafast and reference images was performed by using the nonparametric Wilcoxon rank sum test. Statistical analysis was performed by using RStudio. Statistical significance was set at P < .05.
RESULTS
A total of 66 patients were included in the study, with 37 women and 29 men (Online Supplemental Data). The mean age was 58.7 ± 19 years. The most common indications for MR imaging were altered mental status (21.2%), suspected stroke (18.2%), headache (13.6%), vertigo (12.1%), transient ischemic attack (9.1%), suspected tumor (7.6%), vision loss (4.5%), seizure (1.5%), and ataxia (1.5%). The mean acquisition time for the reference brain MR protocol was 10 minutes, compared with 2:06 minutes for the ultrafast brain MR protocol.
There was agreement on the categorically defined main clinical diagnosis in all but 1 case (98.5% agreement between the 2 protocols). The main categorical diagnosis of the 66 examinations after reviewing both the ultrafast and reference protocol images was no acute findings for 71.2% of cases, acute to subacute infarct for 10.6% of cases, intracranial hemorrhage for 6.1% of cases, intracranial mass lesion for 6.1% of cases, old infarct for 3.0% of cases, and Chiari type I malformation for 3.0% of cases. The ultrafast MS-EPI protocol provided excellent image quality for visualization of the various clinical pathologies encountered.
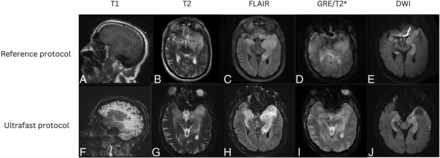
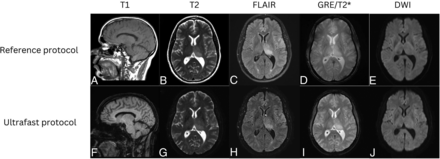
On the single case with discrepant main diagnosis by the 2 reviewing neuroradiologists, there was punctate focus of DWI hyperintensity that may represent acute to subacute infarct that was less conspicuous on the ultrafast DWI (Fig 1). After further reviewing this case with a third adjudicating neuroradiologist, the discrepancy in finding may have been due to a combination of difference in image quality between the 2 protocols and slight differences in section positioning leading to partial volume contamination as the lesion was likely smaller than the section thickness.
A punctate focus of restricted diffusion was less conspicuous on the ultrafast DWI image (left) compared with the reference DWI image (right). The decreased conspicuity may be due to a combination of differences in image quality between the 2 protocols and section positioning leading to partial volume averaging.
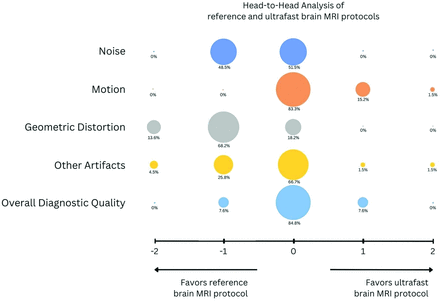
In a head-to-head comparison of image quality between the ultrafast and the reference brain MR protocols, the reference protocol was preferred by the evaluating neuroradiologists in terms of image noise and geometric distortion (P < .05 for both; Fig 2). The ultrafast ms-EPI protocol was preferred over the reference protocol in terms of motion artifacts (P < .01). There was no significant difference between the 2 protocols in terms of overall diagnostic quality (Fig 2).
Bubble plot shows head-to-head comparison between the reference brain MR protocol and the ultrafast ms-EPI protocol. Negative scores indicate preference of the clinical reference protocol; 0 indicates equivalence between the 2 protocols; and positive scores indicate preference of the ultrafast ms-EPI protocol. The reference protocol was preferred by the evaluating neuroradiologists in terms of image noise and geometric distortion (P < .05 for both). The ultrafast protocol was preferred over the reference protocol in terms of motion artifacts (P < .05). There was no significant difference between the 2 protocols in terms of overall diagnostic quality.
Representative images from 4 clinical cases are shown in Figures 3⇓⇓–6, demonstrating similar diagnostic quality between the ultrafast brain MR protocol images and the reference brain MR protocol images. Figure 3 shows a case of amyloid angiopathy with scattered foci of microhemorrhages. There is blurring of the findings on the reference GRE sequence due to motion artifact, and the foci of susceptibility signal are better appreciated on the ultrafast T2* images. Figure 4 shows a case of herpes simplex virus encephalitis with T2/FLAIR hyperintensity and restricted diffusion in the left greater than right mesial temporal lobes, visualized equally well on both the reference and the ultrafast brain MR protocols. Figure 5 shows a case of punctate subacute infarct in the left centrum semiovale on a background of chronic small vessel ischemic disease, visualized equally well on both the reference and the ultrafast brain MR protocols. Figure 6 shows a case of glioma involving the left thalamus with both the reference and the ultrafast brain MR protocol demonstrating ill-defined T2/FLAIR hyperintensity centered in the left thalamus.
Patient with a diagnosis of amyloid angiopathy. Reference sagittal T1-weighted (A), axial T2-weighted (B), FLAIR (C), SWI (D), and DWI (E) show scattered foci of susceptibility signal in the left greater than right frontal lobes consistent with chronic microhemorrhages. The findings were more conspicuous on the ultrafast sagittal T1-weighted (F), axial T2-weighted (G), FLAIR (H), SWI (I), and DWI (J) because of motion artifact on the reference MR images.
Patient with a diagnosis of herpes simplex virus encephalitis. Reference sagittal T1-weighted (A), axial T2-weighted (B), FLAIR (C), SWI (D), and DWI (E) show prominent T1 hypointensity and T2/FLAIR hyperintensity in the left greater than right mesial temporal lobes with scatter foci of susceptibility signal in the left temporal lobe consistent with microhemorrhages. There was associated restricted diffusion in the left greater than right mesial temporal lobes. These signal abnormalities were all seen with similar conspicuity on the ultrafast sagittal T1-weighted (F), and axial T2-weighted (G), FLAIR (H), SWI (I), and DWI (J).
Patient with punctate subacute infarct in the left corona radiata. Reference sagittal T1-weighted (A), axial T2-weighted (B), FLAIR (C), SWI (D), and DWI (E) show a punctate focus of restricted diffusion with associated FLAIR hyperintensity in the left centrum semiovale on a background of white matter T2/FLAIR hyperintensities that likely represent chronic small vessel ischemic disease. The same findings were seen on the ultrafast sagittal T1-weighted (F), axial T2-weighted (G), FLAIR (H), SWI (I), and DWI (J).
Patient with a low-grade left thalamic glioma. Reference sagittal T1-weighted (A), and axial T2-weighted (B), FLAIR (C), SWI (D), and DWI (E) show an ill-defined, mildly expansile, T1 hypointense, and T2/FLAIR hyperintense lesion centered in the left thalamus, compatible with diagnosis of low-grade glioma. Similar findings were appreciated on the ultrafast sagittal T1-weighted (F), axial T2-weighted (G), FLAIR (H), SWI (I), and DWI (J).
DISCUSSION
There has been growing interest in accelerated brain MR imaging techniques in recent years, especially for vulnerable patient populations, such as pediatric patients and those presenting with acute symptoms in the emergency and inpatient settings. These patient populations are often motion prone and are in settings where timely diagnosis is critical to guiding treatment, management, and disposition on expedited time scales. In our study, we found that the 2-minute ultrafast ms-EPI brain MR protocol, which was 8 minutes (80%) faster than the reference protocol, provided similar overall diagnostic image quality and was concordant with the reference brain MR imaging protocol for the main clinical diagnosis in all but 1 case (98.5%).
Other brain MR acceleration techniques, including single-shot EPI and previously reported variations, such as multicontrast EPI (EPIMix), have been reported.9,10 However, at high acceleration factors (R > 2), these techniques can suffer from increased noise, geometric distortion, and residual aliasing artifacts.18,19 A major limitation of single-shot echo-planar FLAIR imaging is the poor tissue contrast between WM and GM. EPIMix, on the other hand, is an ultrafast brain imaging technique by using single-shot EPI to rapidly acquire multiple contrasts.11,12,20 ms-EPI uses an interleaved EPI method that utilizes multiple excitations, resulting in significantly reduced geometric distortion and higher SNR than single-shot-EPI.14⇓-16 The proposed multishot EPI approach employed in this paper requires slightly greater scan time (because of the acquisition of multiple shots) with the expected trade-off of improved image quality in the form of increased SNR and reduced image distortion compared with a single-shot EPI approach, including EPIMix. Single-shot EPI may still have a valuable role in extremely motion-prone patients, or when extreme scan speed is desired.
The ultrafast brain protocol used in this study is based on the ms-EPI technique with a machine-learning assisted reconstruction framework that was previously described by Clifford et al.17 Despite its inherently increased sensitivity to susceptibility-induced variations of the magnetic field, EPI has been previously suggested as an alternative to TSE-based T2-weighted imaging in the clinical setting.16,21 There are some known limitations to the technique, including signal loss of the extracranial soft tissues and mild geometric distortion in the temporal regions, which are inherent to EPI and are partly mitigated by the multishot acquisition. As expected, the reviewing neuroradiologists rated overall image quality, image noise and geometric distortion to be significantly better in the reference protocol images compared with the ultrafast protocol images. More importantly, however, the overall diagnostic quality of the ultrafast protocol was not significantly worse and was considered to be equivalent compared with the reference standard. In the acute clinical settings, such as the emergency department and the inpatient wards, time to diagnosis, treatment, and management directly correlate with clinical outcomes, including mortality and morbidities, for multiple neurologic conditions.22 In addition to patient outcomes, a delay in diagnosis and management also adds to the burden of the hospital systems as it may increase length of hospitalization, the need for increased hospital capacity, and the need for additional health care services.23 Thus, with equivalent overall clinical diagnostic utility, the ultrafast brain MR imaging may be an appropriate first imaging examination of choice to exclude life-threatening and emergent neurologic pathology.
This study was focused on patients with acute neurologic symptoms and adds to a growing body of literature evaluating ultrafast MR imaging protocols in the brain. A recently published study evaluating patients with acute stroke imaged with a deep-learning enhanced 2-minute multicontrast EPI examination found that characterization of patients with stroke by using this protocol was equivalent to the reference sequences.24 Another recent study showed that a deep-learning assisted ultrafast multishot EPI examination implemented at 1.5T was effective in detecting acute intracranial pathology.25 Patients evaluated in the acute setting may have underlying chronic or nonemergent intracranial pathology that would require additional follow-up and consultation. Further evaluation is needed to determine the efficacy of the ultrafast brain MR protocol for detecting nonacute intracranial findings. Furthermore, by greatly accelerating MR acquisition across different contrasts, there is a trade-off of limited evaluation of extracranial structures. This needs to be taken into account when evaluating ms-EPI and clinical suspicion for extracranial processes will require further evaluation with standard MR protocols.
In our study, there was only 1 case (of 66) where there was a finding (punctate focus of cortical restricted diffusion) that was not as well visualized on the ultrafast brain protocol but was seen on the reference protocol. Upon further review, the finding may have been due to differences in section positioning and section thickness (4 mm for the reference DWI and 5 mm for the ultrafast DWI), leading to partial volume contamination. This does raise the question of the sensitivity of the ultrafast MR protocol at picking up small findings and whether the acquisition acceleration to exclude emergent pathology outweighs the slightly lower sensitivity at detecting small findings, which most likely have little impact on clinical care. Future studies with a larger patient population with a wide range of imaging findings is needed to provide a more detailed assessment of the ultrafast brain protocols.
The accelerated acquisition time also benefits from reduced sensitivity to patient motion as the overall acquisition time is shorter. In 11 cases (16.7%), there was less motion on the ultrafast brain protocol images than on the reference protocol images. In patients with altered mental status who do not have the ability to hold still, the ultrafast brain MR protocol may provide superior evaluation compared with the reference protocol, which may be rendered nondiagnostic from motion artifacts. A limitation of the reduced sampling of the ultrafast brain protocol ms-EPI sequences is that, if motion were to occur in between shots, motion artifacts may be inadvertently exacerbated from intershot phase error. Motion-correction techniques, such as navigator-based prospective motion correction or scout accelerated motion estimation and reduction techniques, may be helpful to address intershot phase errors with minimal impact on overall acquisition time.26,27
Pediatric patients are an important population for whom motion during MR imaging is a concern, especially infants and very young children who may have difficulty remaining still for extended periods. General anesthesia may be required for challenging patients, but the use of anesthetics in pediatric patients has known potential negative effects, including nausea, vomiting, and disorientation, or more serious adverse events, such as cardiorespiratory depression.28 In the acute clinical setting, institutions may implement abbreviated MR protocols to avoid sedation that includes only a single contrast, usually T2-weighted imaging.29⇓-31 The sensitivity of abbreviated MR protocols, however, is limited with 1 study reporting 14% of cases had undetected findings on a fast-brain MR imaging protocol.32 An ultrafast 1-minute pediatric brain protocol has been previously proposed, which uses optimized faster versions of commercially available sequences.33 While the preliminary image quality was good in a previously proposed ultrafast MR protocol, there was no direct evaluation of the final clinical diagnosis. Accelerated protocols, however, have a limited role in sedated patients as the patient is already under anesthesia and standard imaging will provide superior image quality.
There are several limitations to this study. First, although the patient sample size is adequate with 66 patients, most did not exhibit any acute intracranial findings. Future investigations need to include a larger sample size comprising patients with acute findings to further determine sensitivity and specificity of the ultrafast brain MR protocol. It will be important to determine the optimal balance between reducing scan time and potential slight loss in sensitivity in detecting abnormal findings. Second, this study only included adult patients in the emergency and inpatient settings. Pediatric patients, as mentioned, may greatly benefit from accelerated MR examinations; therefore, it will be important to further assess the efficacy of the ultrafast brain MR protocol in this population. Third, this study was conducted exclusively in acute clinical settings. The ultrafast brain MR protocol may also benefit patients who are claustrophobic or unable to tolerate long MR examinations for various reasons. Fourth, given the inherent differences in image characteristics of the reference and ultrafast MR imaging protocols, true blinding was difficult to achieve for side-by-side comparisons. This is unavoidable but does represent real-life situations when new sequences are introduced clinically and radiologists are asked to evaluate the quality of the new sequence compared with the reference. Last, the neuroradiologist reviewers assessed the ultrafast brain MR protocol and the reference brain MR protocol in succession rather than by randomized evaluation. This method is more stringent on the ultrafast brain MR protocol as the reference brain MR protocol is reviewed after the ultrafast brain MR protocol to identify any missed findings.
CONCLUSIONS
The 2-minute ultrafast brain MR imaging protocol provides high accuracy for evaluation of acute pathology in emergency department and inpatient settings. While there was greater image noise and geometric distortion on the ultrafast brain MR protocol images compared with the reference protocol, there was reduced motion artifact with similar overall diagnostic quality.
Footnotes
Supported by U.S. Department of Health and Human Services, National Institutes of Health, P41EB030006, RSNA Research and Education Foundation, Seed Grant, Siemens Healthineers.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
REFERENCES
- Received July 17, 2023.
- Accepted after revision December 7, 2023.
- © 2024 by American Journal of Neuroradiology