Abstract
BACKGROUND AND PURPOSE: The impairment of the glymphatic system, a perivascular network crucial for brain waste clearance, has been linked to cognitive impairment, potentially attributed to the accumulation of brain waste. Although marijuana use has been associated with poorer cognitive performance, particularly in adolescents, its influence on the glymphatic system remains unexplored. This study evaluated the influence of the age of first marijuana use and the total number of lifetime uses on the glymphatic system, measured using the index of DTI along the perivascular space (DTI-ALPS). Furthermore, we explored the correlation between glymphatic clearance and cognitive performance among marijuana users.
MATERIALS AND METHODS: In this study, 125 individuals who reported using marijuana at least once in their lifetime (43 men; mean age, 28.60 [SD, 3.84] years) and 125 individuals with zero lifetime cannabis use (nonusers; 44 men; mean age, 28.82 [SD, 3.56] years) were assessed. ALPS indices of all study participants were calculated using 3T diffusion MR imaging data (b = 1000 s/mm2).
RESULTS: After we adjusted for age, sex, education years, Pittsburgh Sleep Quality Index, alcohol use, tobacco use, and intracranial volume, our analysis using a univariate General Linear Model revealed no significant difference in the ALPS index among nonusers and marijuana users with different ages of first use or various frequencies of lifetime usage. However, in marijuana users, multiple linear regression analyses showed associations between a lower ALPS index and earlier age of first marijuana use (standardized β, −0.20; P = .041), lower accuracy in the working memory 0-back task (standardized β, 0.20; P = .042), and fewer correct responses in the Fluid Intelligence Test (standardized β, 0.19; P = .045).
CONCLUSIONS: This study shows the potential use of DTI-ALPS as a noninvasive indirect indicator of the glymphatic clearance in young adults. Our findings show novel adverse effects of younger age at first use of marijuana on the glymphatic system function, which is associated with impaired working memory and fluid intelligence. Gaining insight into the alterations in glymphatic function following marijuana use could initiate novel strategies to reduce the risk of cognitive impairment.
ABBREVIATIONS:
- ALPS
- along the perivascular space
- CB1
- type-1 cannabinoid
- FA
- fractional anisotropy
- HCP
- Human Connectome Project
- ICV
- intracranial volume
- ISF
- interstitial fluid
- MLR
- multiple linear regression
- PSQI
- Pittsburgh Sleep Quality Index
- SE
- standard error
- SSAGA
- Semi-Structured Assessment for the Genetics of Alcoholism
- VIF
- variance inflation factor
Summary
PREVIOUS LITERATURE:
Alterations of the glymphatic system, a perivascular network essential for clearing brain waste, has been linked with cognitive impairment, possibly because of waste accumulation. Concurrently, marijuana use has shown associations with poorer cognitive performance, particularly in adolescents; however, its impact on the glymphatic system has not yet been investigated.
KEY FINDINGS:
In marijuana users, multiple linear regression analyses showed significant associations between a lower ALPS index and earlier age of first marijuana use, lower accuracy in the working memory 0-back task, and fewer correct responses in the Fluid Intelligence Test.
KNOWLEDGE ADVANCEMENT:
Our findings indicated that in young adults, dysfunction in glymphatic clearance, as measured by the ALPS index correlated with an earlier initiation of marijuana use. Furthermore, the glymphatic dysfunction in marijuana users is associated with deficits in working memory and fluid intelligence.
Marijuana is currently the most extensively used psychoactive substance globally, partly due to shifts in legal and societal attitudes toward its use.1 In 2020, approximately 209 million individuals 15–64 years of age reported past-year marijuana use.2 The past-year prevalence of marijuana use is higher among adolescents compared with adults.2 Marijuana use during the crucial neurodevelopment period of adolescence may lead to alterations in brain structure and function.3,4 Consequently, regular marijuana use during adolescence is correlated with a higher risk of adverse outcomes, including cognitive impairment and mood disorders (ie, anxiety and depression).5,6 A recent systematic literature review on diffusion MR imaging studies involving marijuana users reported a notable lower WM integrity, particularly in the superior longitudinal fasciculus and corpus callosum.7
Studies suggest a close association between glymphatic system dysfunction and cognitive impairment, as well as mood disorders.6,8,9 The glymphatic, or glia-lymphatic system, has gained recent recognition as a brain waste clearance mechanism.10 According to the glymphatic hypothesis, the pulsation of the arterial walls propels CSF through aquaporin-4 channels in astrocytic endfeet, along perivascular spaces, and into the brain parenchyma. This influx of CSF into the parenchyma subsequently facilitates the movement of interstitial fluid (ISF) and metabolic waste toward the perivenous spaces surrounding the deep veins.11 Impairment of the glymphatic clearance system has a direct effect on brain waste accumulation, contributing to cognitive decline.12,13 The glymphatic system has also been shown to play a role in maintaining WM integrity.14 Therefore, alterations in glymphatic clearance could potentially impact the health of the WM structure.
MR imaging using intrathecal contrast agents, such as gadolinium-based contrast agents, allows the evaluation of the glymphatic system in humans.15 However, this method is relatively invasive, and gadolinium accumulation in the brain has raised concerns,16 limiting its clinical application. A more recent approach, DTI along the perivascular space (DTI-ALPS), based on diffusion MR imaging, has emerged as a promising noninvasive method for studying the human glymphatic system.17,18 The DTI-ALPS method yields the ALPS index, representing the ratio of diffusivity along the perivascular space surrounding the deep medullary vein at the level of the lateral ventricle body to the diffusivity in a direction perpendicular to the major fiber tracts.17,18 At the level of the lateral ventricle body, the medullary veins run perpendicular to the ventricular wall, paralleling the direction of the perivascular space, whereas the medullary arteries and veins, as vessels of the brain parenchyma, accompany the perivascular space, which is a principal drainage pathway of the glymphatic system. Meanwhile, the projection fibers run in the cranial-caudal direction, and the association fibers run in the anterior-posterior direction. Thus, in this region, the perivascular space is perpendicular to both the association and projection fibers.18 Although the ALPS index is a noninvasive, indirect measure of the human glymphatic system, a previous study has showed a strong correlation with the glymphatic clearance function, as assessed through glymphatic MR imaging following intrathecal gadolinium administration, the current criterion standard evaluation of the human glymphatic system,19 validating the use of the ALPS index.
Reductions in the ALPS index have been noted in older adults at risk of dementia and in individuals with Alzheimer disease.9,18,20 This observation implies that the decrease in the ALPS index could be attributed to the accumulation of amyloid-β, potentially due to a deficiency in glymphatic clearance.9,18,20 ALPS index studies have mainly been conducted on older adult cohorts; however, recently, studies have applied the ALPS index to younger age groups. For example, a lower ALPS index has been observed in patients with juvenile myoclonic epilepsy (age range, 12−46 years)21 and in patients with acute lymphoblastic leukemia (age range, 4−16 years).22
Alterations of the glymphatic system, a perivascular network essential for clearing brain waste, has been linked to cognitive impairment, possibly because of waste accumulation. Concurrently, marijuana use has shown associations with poorer cognitive performance, particularly in adolescents; however, its impact on the glymphatic system has not yet been investigated. We hypothesized that marijuana use could influence the glymphatic system and is correlated with cognitive decline. Our study using the ALPS index explored the influence of the age of first marijuana use and the number of lifetime uses on the glymphatic system. Furthermore, we evaluated the association between the ALPS index and cognitive performance in recreational marijuana users from the Washington University–University of Minnesota (WU-Minn) Human Connectome Project (HCP) consortium.23 Gaining a deeper understanding of the alterations of glymphatic clearance following marijuana use could initiate strategies to reduce the risk of cognitive decline.
MATERIALS AND METHODS
Study Participants
The data analyzed in this study were obtained from the S1200 data set of the WU-Minn HCP consortium (https://www.humanconnectome.org/storage/app/media/documentation/s1200/HCP_S1200_Release_Reference_Manual.pdf).23 The inclusion criteria of study participants (Fig 1) are provided in the Online Supplemental Data.
Flow chart of inclusion and exclusion criteria, along with the count of individuals meeting the criteria and included in the study population. MMSE indicates the Mini-Mental State Examination; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition.
Marijuana Use
Following previous studies,1,24⇓⇓⇓-28 we used “lifetime use” to categorize the study participants as nonusers (individuals with zero lifetime cannabis use) and users (those who reported using marijuana at least once in their lifetime). Marijuana use was assessed using self-report measures obtained through the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA). The number of times used was categorized as follows: 0 (never used), 1 (1–5 uses), 2 (6–10 uses), 3 (11–100 uses), 4 (101–999 uses), or 5 (≥1000 uses). The SSAGA categorized the age of first use as the following: 1 (14 years of age or younger), 2 (15 − 17 years of age), 3 (18 − 20 years of age), 4 (21 years of age or older), or 5 (never used). In this study, the age of first use was reverse-scored (0 [never used], 1 [21 years of age or older], 2 [15 − 17 years of age], 3 [18 − 20 years of age], 4 [14 years of age or younger]), yielding higher scores to indicate an earlier age of first use, aligning with the frequency of use measure.
Tobacco and Alcohol Use
The criteria of tobacco and alcohol use are provided in the Online Supplemental Data.
Imaging Data Acquisition and Preprocessing
Imaging data were acquired using a HCP-customized 3T Connectome Magnetom Skyra magnet (Siemens).29 DWIs downloaded from the HCP portal underwent a minimal preprocessing pipeline.30 To calculate the ALPS index, we obtained the DWIs with 2 b-values, 0 and 1000 s/mm2, extracted from the preprocessed data set. Further details of DWIs are provided in the Online Supplemental Data.
Calculation of the ALPS Index
The ALPS index was calculated using a validated semiautomated pipeline.31 The fractional anisotropy (FA) map of all individuals was initially registered into the FMRIB58_FA standard space (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FMRIB58_FA) using both linear and nonlinear transformations. For ROI placement, 1 subject with the smallest degree of warping was selected. Using this subject’s color-coded FA map, we placed spherical ROIs measuring 5 mm in diameter in the projection and association areas at the level of the bilateral lateral ventricle body (Fig 2). The resulting ROIs were then registered to the same FA template, and the position of the ROIs was visually confirmed for each participant. If necessary, a manual correction was performed by slightly adjusting the ROIs.
Calculation of the DTI index along the perivascular space (ALPS index). A, Color-coded FA map shows the distribution of projection fibers (blue, z-axis) and association fibers (green, y-axis) at the lateral ventricle level of the body. Spherical ROIs measuring 5 mm in diameter were positioned in projection and association areas. The perivascular space runs perpendicular to the projection and association tracts. B, Diffusivity maps in the x-axis (Dxx), y-axis (Dyy), and z-axis (Dzz). C, The ALPS index was derived from the ratio of the mean x-axis diffusivity in the projection area (Dxxproj) and x-axis diffusivity in the association area (Dxxassoc) to the mean of y-axis diffusivity in the projection area (Dyyproj) and z-axis diffusivity in the association area (Dzzassoc).
In the projection fibers area, the main fibers run along the z-axis direction, while the x-axis and y-axis are perpendicular to the main fibers. Moreover, in the association fibers area, the main fibers run in the direction of the y-axis, and the x-axis and z-axis are perpendicular to the main fibers. Therefore, the ALPS index was derived from the ratio of the average values of the x-axis diffusivity in the projection fibers area (Dxxproj) and the x-axis diffusivity in the association fibers area (Dxxassoc) to the average values of the y-axis diffusivity in the projection fibers area (Dyyproj) and the z-axis diffusivity in the association fibers area (Dzzassoc), as shown below:

The average values of left and right ALPS indices were evaluated. An ALPS index closest to 1 reflects minimal diffusion along the perivascular space, whereas a larger ratio indicates larger water diffusivity along the perivascular space.18
Furthermore, we extracted FA values, which serve as an index of WM integrity in the association and projection fibers, using identical ROIs. Subsequently, the statistical analyses incorporated the average FA values of both the left and right projection and association fibers.
Intracranial Volume Measurements
The intracranial volume (ICV) was obtained for each study participant and was used as a covariate in the statistical analyses. The details of ICV measurements are provided in the Online Supplemental Data.
Statistical Analysis
Statistical analyses were conducted with SPSS Statistics Version 27 for Macintosh (IBM). The normality of the data was assessed using the Kolmogorov-Smirnov test. Demographic and clinical data of nonusers and users were analyzed using independent samples t tests for normally distributed continuous data or the Mann-Whitney U tests for non-normally distributed continuous data. For categoric variables, the χ2 test was used. In all analyses, statistical significance was set at P (2-tailed) < .05.
The ALPS index was compared among nonusers and marijuana users with different ages of first use (nonuser group versus user group 1 [14 years of age or younger] versus group 2 [15−17 years of age] versus group 3 [18−20 years of age] versus group 4 [21 years of age or older]) or those with various frequencies of lifetime usage (nonuser group versus user group 1 [1–5 uses] versus group 2 [6–10 uses] versus group 3 [11–100 uses] versus group 4 [101–999 uses] versus group 5 [≥1000 uses]) using a univariate General Linear Model with a Bonferroni correction to adjust for multiple group comparisons. This analysis controlled for age, sex, years of education, tobacco use, alcohol use, the Pittsburgh Sleep Quality Index (PSQI) score, and the ICV (model 1). Marijuana use has been associated with loss of WM integrity, including in the association and projection fibers.7 Therefore, the average FA values, an index of WM integrity,32 measured in the ROIs of the association and projection fibers were included as control variables in the evaluation of the ALPS index, in addition to the covariates in model 1 (model 2).
Multiple linear regression (MLR) analysis was used to model the associations between the ALPS index (dependent variable) and the age of first marijuana use or number of lifetime uses. MLR analysis was also conducted to identify associations between the ALPS index and cognitive assessment scores. The multicollinearity of predictor variables was addressed by assessing variance inflation factor (VIF) values, with VIF values ≤5 indicating the absence of multicollinearity.33 Furthermore, we performed partial correlation tests to assess relationships between the ALPS index and variables, including the age of first marijuana use, lifetime use count, and cognition scores. In linear regression and partial correlation analyses, the same covariates as those included in the between-group comparison analyses (models 1 and 2) were used.
Additionally, we compared the average FA values measured in the ROIs of the association and projection fibers between nonusers and users with different ages of first use or those with various lifetime usage frequencies using a univariate General Linear Model, while controlling for age, sex, years of education, tobacco use, alcohol use, PSQI score, and ICV. Moreover, we performed partial correlation analysis to examine the association between the ALPS index and ICV across all subjects, while controlling for age, sex, years of education, tobacco use, alcohol use, and the PSQI scores as covariates.
RESULTS
Study Participants’ Demographics, Clinical Characteristics, and Substance Use
In this study, we analyzed 250 subjects with an age range of 22–37 years, including 125 marijuana recreational users (43 men and 82 women; mean age, 28.60 [SD, 3.84] years) and 125 nonusers (44 men and 81 women; mean age, 28.82 [SD, 3.56] years). The demographic and clinical characteristics of participants are summarized in the Online Supplemental Data.
No differences were observed between nonusers and marijuana users in age, sex, years of education, body mass index, systolic and diastolic blood pressures, as well as the Mini-Mental State Examination scores. Marijuana users had significantly higher PSQI scores (P = .022) and significantly higher (P < .001) alcohol and tobacco usage. Additionally, marijuana users had significantly lower ICV compared with nonusers (P = .033).
Marijuana users had significantly lower correct responses (P = .010) and reaction times (P = .011) in the Fluid Intelligence Test compared with nonusers. No significant differences were observed in other cognitive test scores (Online Supplemental Data).
The Evaluation of ALPS Index
Between-Group Differences in ALPS Index.
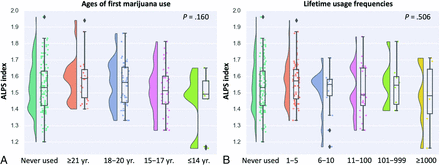
We found no significant differences in the ALPS index among nonusers and marijuana users with different ages of first use (model 1 [P = .16], model 2 [P = .15]; Fig 3A). Furthermore, we also observed no significant differences in the ALPS index among nonusers and marijuana users with various lifetime usage frequencies (model 1 [P = .506], model 2 [P = .488]; Fig 3B).
Raincloud plots of ALPS indices (A) among nonusers (individuals with zero lifetime cannabis use) and users (those who reported using marijuana at least once in their lifetime) with different ages of first use and (B) among nonusers and users with various lifetime usage frequencies. The P values correspond to a univariate General Linear Model after controlling for age, sex, years of education, tobacco use, alcohol use, PSQI score, and intracranial volume.
Associations between the Age of First Marijuana Use or the Number of Uses in a Lifetime and the ALPS Index in Marijuana Users.
We observed that a lower ALPS index was associated with younger ages at first marijuana use in both linear regression (model 1: standardized β, −0.20 [standard error, SE: 0.016]; P = .041; VIF = 1.22; model 2 [standardized β, −0.20; SE: 0.016]; P = .037; VIF = 1.23) and partial correlation (model 1 [r = −0.19; P = .041], model 2 [r = −0.19; P = .037]) analyses. However, no significant association was found between the ALPS index and the number of marijuana uses in both the linear regression (model 1 [standardized β, −0.090; SE: 0.011]; P = .36; VIF = 1.25; model 2 [standardized β, −0.088; SE: 0.011]; P = .37; VIF = 1.25) and partial correlation (model 1 [r = −0.086; P = .36], model 2 [r = −0.084; P = .37]) analyses.
Associations between Cognitive Performance and the ALPS Index in Marijuana Users.
A lower ALPS index was significantly associated with lower accuracy in the working memory 0-back task in MLR (model 1 [standardized β, 0.20; SE: 0.001]; P = .042; VIF = 1.27; model 2 [standardized β, 0.22; SE: 0.001]; P = .030; VIF = 1.32) and partial correlation (model 1 [r = 0.19; P = .041], model 2 [r = 0.20; P = .030]) analyses. Additionally, a lower ALPS index was associated with fewer correct responses in the Fluid Intelligence Test in MLR (model 1 [standardized β, 0.19; SE: 0.003]; P = .045; VIF = 1.16; model 2 [standardized β, 0.21; SE: 0.003]; P = .031; VIF = 1.20) and partial correlation (model 1 [r = 0.19; P = .040]; model 2 [r = 0.21; P = .031]) analyses. These findings suggest subtle-but-substantial effects of marijuana use on working memory and fluid intelligence.
Additional Analyses
Between-Group Differences in FA.
We found no significant differences in FA values among nonusers and marijuana users with different ages of first use (P = .866) or those with various lifetime usage frequencies (P = .742).
Association between the ALPS Index and ICV.
No significant association was observed between the ALPS index and ICV (r = −0.11; P = .257).
DISCUSSION
The present study has expanded the potential applications of diffusion MR imaging–based measures, including the ALPS index, as noninvasive, indirect indicators of the glymphatic system in young adults. Our results provide evidence of the adverse effects of younger age at first use of marijuana on the glymphatic system function, as indexed by the ALPS index. Furthermore, associations were observed between a lower ALPS index and impaired working memory and fluid intelligence in marijuana users.
Our findings suggest that an earlier age of first marijuana use is linked to potential impairment of the glymphatic system, indicated by a lower ALPS index. A lower ALPS index appears to signify reduced water diffusivity within the perivenous spaces, possibly due to compromised glymphatic clearance flow, specifically, a reduction in CSF-ISF drainage toward the perivenous space.18 However, group comparison revealed no significant differences in the ALPS index among nonusers and marijuana users with different ages of first marijuana use or those with various lifetime usage frequencies. This observation could imply that the marijuana use in our study sample might not be severe enough to yield noticeable effects. Our findings align with a prior study assessing HCP data, indicating that an earlier onset of marijuana use was associated with lower WM coherence.1 They found no significant differences in WM between marijuana users and nonusers, similar to our results.1 Furthermore, these negative findings may be because of the imbalanced number of subjects in user subgroups, particularly the limited number of younger age first-time marijuana users and those with a higher frequency of lifetime use, possibly reducing the statistical power. Future studies should aim to include a larger and more balanced number of subjects in each user subgroup.
The early initiation of substance use has been observed to impact the developing adolescent brain, including the endocannabinoid system.34 Δ9-tetrahydrocannabinol, the primary psychoactive component of cannabis, acts as an agonist of the type 1 cannabinoid (CB1) receptor.35 CB1 receptors are expressed in astrocytes, and mounting evidence suggests that persistent activation of mouse astroglial CB1 receptors associated with mitochondria disrupts glucose metabolism and lactate production in the brain. Consequently, this disturbance leads to changes in neuronal activity and impaired cognitive function.36 Astrocytes play a crucial role in the glymphatic system by creating perivascular spaces with their vascular endfeet around the cerebral vasculature.37 Thus, it is possible that marijuana use disrupts glymphatic flow by affecting astrocyte function, warranting further investigation.
The use of cannabis, especially at an early age and with frequent usage, has been associated with an increased risk of cognitive impairment.38,39 Consistent with a previous study,40 marijuana users in our cohort exhibited significantly lower Fluid Intelligence Test scores compared with nonusers, indicating a reduced ability to think logically and solve problems in novel, unfamiliar situations. We also observed a correlation between a lower ALPS index and a decrease in the number of correct responses in the Fluid Intelligence Test. Furthermore, despite no difference between marijuana users and nonusers, we identified a correlation between a lower ALPS index and a decrease in accuracy on the working memory test. Early investigations of working memory in marijuana users have indicated that acute cannabis use is associated with impairment in holding, manipulating, and remembering information.41 A study comparing groups based on amyloid-β status found a more significant decline in fluid intelligence among those in the amyloid-β-positive group compared with the amyloid-β-negative group.42 However, this study involved older adults. On the basis of our findings, further research should evaluate amyloid-β deposition in young adults, particularly in marijuana users. Δ9-tetrahydrocannabinol has been suggested as the primary contributor to working memory deficits linked to the duration of cannabis use.41 Taken together, our findings suggest that cognitive impairment related to marijuana use might be linked to reduced clearance of brain waste products (eg, amyloid-β) due to glymphatic system dysfunction, potentially associated with the activation of CB1 receptors.
Our findings revealed a significant difference in ICV between marijuana users and nonusers, with marijuana users having lower ICV values. Most interesting, prior studies have consistently reported no significant differences in ICV between individuals who use marijuana and those who do not.43,44 Consequently, we assumed that the observed lower ICV values among marijuana users may be attributed to individual variation. To account for the potential influence of ICV on the evaluation of the ALPS index, we incorporated ICV as a covariate in all statistical analyses. Furthermore, we assessed the correlation between ICV and the ALPS index and found no significant results. However, a previous study suggested that marijuana use during adolescence is linked to reduced cortical thickness, particularly in the prefrontal regions.28 In our future research, we will explore the correlation between the ALPS index and regional cortical thickness to gain further insight.
The present study has some limitations. First, it is a cross-sectional study. To assess changes in the marijuana-related ALPS index, future studies should use longitudinal data from larger population samples with a balanced representation of marijuana usage levels. Second, the study did not use MR imaging–based tracers, considered the current criterion standard for assessing glymphatic function in humans.17 However, the ALPS index showed a high correlation with glymphatic clearance function, as determined through glymphatic MR imaging following intrathecal administration of gadolinium.14 Third, the ALPS index does not solely assess the diffusivity of the perivenous space surrounding the deep medullary vein; it is also influenced by the adjacent WM microstructure within the ROI. As a result, the interpretation of the ALPS index requires careful consideration and warrants additional investigation. However, the significant associations between the ALPS index and younger ages at first marijuana use or cognitive performance were maintained even after including FA as a covariate in model 2. Furthermore, we did not observe significant changes in FA between nonusers and users with different ages of first use or various levels of lifetime usage. These findings suggest that the ALPS index measurements in marijuana users identified in this study were primarily attributed to changes in water diffusivity in the perivenous space and were less likely affected by alterations in WM integrity. However, considering that marijuana users have shown reduced WM integrity and given the known role of the glymphatic system in maintaining this integrity, further research is warranted to explore the potential link between the integrity of individual WM tracts and glymphatic clearance in marijuana users. Finally, this study focused only on the ALPS index; however, there are some alternative methods for evaluating the human glymphatic system, each with its own advantages and disadvantages, as recently nicely summarized by Kamagata et al.17
CONCLUSIONS
Our findings show that a lower ALPS index value was correlated with an earlier age of first marijuana use. The lower ALPS index observed in recreational marijuana users may indicate a potential link between marijuana use and glymphatic function impairment. This study suggests associations between glymphatic dysfunction in marijuana users and deficits in working memory and fluid intelligence. However, no significant differences in the ALPS index were observed among nonusers and marijuana users with different ages of first marijuana use or those with various lifetime usage frequencies. The lack of significant findings might be because of the nature of marijuana use in our study sample, which may not have been severe enough, and the imbalanced number of subjects in user subgroups. Future studies should aim to include subjects with heavier use and a more balanced number of subjects in each user subgroup. Finally, it is important to exercise caution when interpreting the results due to the limitations of this study.
Footnotes
This study was supported by the Juntendo Research Branding Project, the Project for Training Experts in Statistical Sciences; the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI; grant Nos. 23H02865 and 23K14927), a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan, and the Brain/MINDS Beyond program of the Japan Agency for Medical Research and Development (grant Nos. JP18dm0307004 and JP19dm0307101), and Agency for Medical Research and Development (grant No. JP21wm0425006).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received November 21, 2023.
- Accepted after revision February 20, 2024.
- © 2024 by American Journal of Neuroradiology