SUMMARY:
The International League Against Epilepsy (ILAE) is an organization of 120 national chapters providing the most widely accepted and updated guidelines on epilepsy. In 2022, the ILAE Task Force revised the prior (2011) classification of focal cortical dysplasias to incorporate and update clinicopathologic and genetic information, with the aim to provide an objective classification scheme. New molecular-genetic information has led to the concept of “integrated diagnosis” on the same lines as brain tumors, with a multilayered diagnostic model providing a phenotype-genotype integration. Major changes in the new update were made to type II focal cortical dysplasias, apart from identification of new entities, such as mild malformations of cortical development and cortical malformation with oligodendroglial hyperplasia. No major changes were made to type I and III focal cortical dysplasias, given the lack of significant new genetic information. This review provides the latest update on changes to the classification of focal cortical dysplasias with discussion about the new entities. The ILAE in 2017 updated the classification of seizure and epilepsy with 3 levels of diagnosis, including seizure type, epilepsy type, and epilepsy syndrome, which are also briefly discussed here.
ABBREVIATIONS:
- BOS
- bottom of the sulcus
- EDGE
- edge-enhancing gradient-echo
- EEG
- electroencephalography
- FCD
- focal cortical dysplasia
- ILAE
- International League Against Epilepsy
- MAP2
- microtubule-associated protein 2
- MEG
- magnetoencephalography
- MCD
- malformations of cortical development
- mMCD
- mild malformations of cortical development
- MOGHE
- malformation with oligodendroglial hyperplasia
- mTOR
- mechanistic target of rapamycin
- NeuN
- neuronal nuclear protein
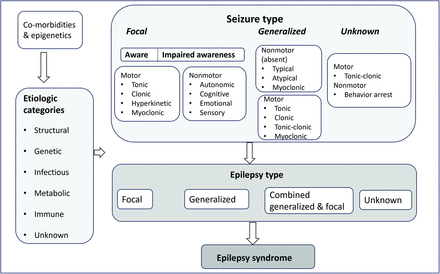
The International League Against Epilepsy (ILAE), founded in 1909, is an organization of >120 national chapters and is the premier international resource for current and emerging standards and best practice parameters in the management of epilepsy. In 2017, the ILAE presented the updated classification of seizures and epilepsy, given the rapid advancement in the understanding of the underlying mechanism, with greater focus on genetics. Epilepsy classification is the key clinical tool in evaluating an individual who is presenting with seizures, and it has significant treatment implications. The new classification has a 3-tier approach of classifying disease into seizure type, epilepsy type, and, finally, the presence of any epilepsy syndrome (Fig 1). This approach acknowledges the wide variability in resources around the world, thereby implying that different levels of classification will be possible, depending on the resources, though diagnosis at all 3 levels is ideal and desirable.1
This diagram highlights the 3-tier approach to classifying disease into seizure type, epilepsy type, and, finally, the presence of any epilepsy syndrome. The major etiologic categories of epilepsy/seizures are also depicted.
In 2022, the ILAE task force published the updated classification of focal cortical dysplasias with new entities based on molecular-genetic characterizations and highlighting the role of phenotype-genotype integration. This relied on significant new information and better knowledge about the clinical, electroencephalography (EEG), and MR imaging phenotype along with the genetic character of these lesions. New categories based on histopathology included mild malformations of cortical development (mMCDs), malformations with oligodendroglial hyperplasia (MOGHE), and no definite focal cortical dysplasia (FCD) on histopathology. The task force also proposed the integrated, multilayered classification scheme for FCD comprising imaging, histopathology, and genetic findings. This multilayered integration improves clarity, paving the way for broader international communication and collaboration in this field. This change includes histopathologic assessment (layer 1), integration of molecular-genetic results (layer 2), neuroimaging findings (layer 3), and the final integrated diagnosis (layer 4).
Histopathologic examination of brain tissue remains the criterion standard for the diagnosis of focal epilepsy. Immunohistochemical staining is mandatory to confirm the diagnosis of FCD, primarily relying on neuronal markers like neuronal nuclear protein (NeuN) and microtubule-associated protein 2 (MAP2). Despite the variability in resources and access to specialized imaging and pathology services across different centers, the ILAE recommends incorporation of available information through a layered approach to the highest extent possible. For the neuroradiologist, the ILAE recommends a detailed anatomic description of abnormal findings on MR imaging (eg, crown of gyrus versus bottom of the sulcus [BOS]) along with details on the magnet strength and protocol used, with the idea that this will help in standardization and multidisciplinary discussion.2⇓-4
Classification of the Epilepsy: Position Paper of the ILAE
Demarcation of seizures from epilepsy is the first important step in the nomenclature. “Seizure is a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain.” Epilepsy is characterized by an enduring predisposition to cause seizures and traditionally defined as “two unprovoked seizures more than 24 hours apart.” This demarcation thereby implies that seizure is an event, and epilepsy is the disease involving recurrent unprovoked seizures. The definition of epilepsy was changed in 2017 by ILAE and is now defined as “either (A) at least 2 unprovoked seizures occurring greater than 24 hours apart; (B) one unprovoked seizure and a probability of further seizures similar to the general recurrence risk, occurring over the next 10 years and finally (C) diagnosis of an Epilepsy Syndrome.” Epilepsy syndrome is defined as “a characteristic cluster of clinical and EEG features, often supported by specific etiological findings (structural, genetic, metabolic, immune, and infectious)” and frequently has an age-dependent presentation.1,5 Epilepsy is supposed to be resolved when the patient has remained seizure-free for the past 10 years and is off antiseizure medicines for at least the past 5 years. The revised framework emphasizes 3 levels of diagnosis (Fig 1), including seizure type, epilepsy type (focal, generalized, combined generalized, and focal, unknown) and epilepsy syndrome. At each level, the underlying etiology should be defined with 6 major etiologic categories identified. After decades of use, many terms like “simple partial” and “complex partial” were discontinued and replaced by more accurate terminology of “focal aware” and “focal seizures with impaired awareness.” New focal and generalized seizure types have been identified along with the addition of an “emotional” component to seizure type.
EEG and neuroimaging form the cornerstone for the etiologic classification at all the 3 levels. The seizure and epilepsy types include focal, generalized, and unknown types with the addition of a new subtype of “combined general and focal” epilepsy. Generalized epilepsy is characterized by generalized spike-wave activity on EEG with a range of seizure types, including absence, myoclonic, atonic, tonic, and tonic-clonic seizures. Focal epileptiform discharges on interictal EEG characterize focal epilepsies, which can be focal aware, focal unaware, focal motor, or nonmotor. The diagnosis of all the types is made on clinical assessment, supported by the EEG findings.1⇓⇓⇓-5
Etiologic Categories
After classification into the seizure/epilepsy type, determination of the underlying etiology is the next major step, because this will significantly guide treatment and prognostication. The etiology of epilepsy was divided into 4 categories: idiopathic, symptomatic, provoked, and cryptogenic by the ILAE in 2011. With newer information and better understanding of the pathogenesis, the ILAE in 2022 identified 6 major categories (Fig 1), including structural, genetic, infectious, metabolic, immune, and unknown. The structural category is the most important type from the neuroradiologist’s point of view because this has the highest chance of abnormal findings on imaging. Examples in this category include cortical malformations, mesial temporal sclerosis, stroke, and neoplasms. Molecular markers and genetics have led to the identification of the causative genetic mutation in many epilepsies, most frequently arising de novo rather than being inherited. This identification is classically seen in infants with severe developmental and epileptic encephalopathies, a great example being Dravet syndrome with SCN1A gene mutation.
Infection is the most common cause for epilepsy worldwide with, however, wide variability in type. Neurocysticercosis, tuberculosis, and HIV are common in developing countries, whereas viral encephalitis is more common in the developed world. Immune-related epilepsy is the category with the most notable expansion during the past decade, primarily driven by greater access to antibody testing and recognition of many new entities, including a wide range of autoimmune encephalitides. There could be >1 etiology for the patient’s epilepsy, a great example being phakomatoses, like tuberous sclerosis, with structural and genetic changes, the former being the target for epilepsy surgery and latter used for genetic counseling. Another example would be polymicrogyria, which is a structural lesion; however, it could be secondary to genetic mutations (AKT3) or infections (intrauterine cytomegalovirus). Neuroimaging in the form of MR imaging is the cornerstone for the identification of the underlying etiology with numerous advances in technique and protocol, highlighted below.1,6
Advances in Neuroimaging
Neuroimaging in the form of MR imaging is the cornerstone of all the epilepsy types and etiologic categories, though it has a more important role in some subtypes compared with others. Classically indicated for focal drug-refractory epilepsies, MR imaging is usually performed in all types, given the considerable clinical overlap. Among the etiologic subcategories, MR imaging more frequently has positive findings in structural, infectious, and immunologic categories compared with genetic and unknown types. Recent advances in neuroimaging include ultra-high-field imaging with a dedicated protocol and newer sequences, arterial spin-labeling. perfusion MR imaging, and simultaneous EEG fMRI. FDG-PET and magnetoencephalography (MEG) (Fig 2) are other new developments increasingly being used. The sensitivity of MR imaging studies can be improved using dedicated epilepsy MR imaging protocols interpreted by subspecialized neuroradiologists. Studies have found that the sensitivity of the general radiologist’s interpretation of standard MR imaging for focal lesions, neuroradiologist’s reports of routine MR imaging, and the neuroradiologist’s reporting of dedicated epilepsy protocol MR imaging were 39%, 50%, and 91%, respectively.7 The ILAE recommends use of the Harmonized Neuroimaging of Epilepsy Structural Sequences (HARNESS MR imaging) protocol with isotropic, millimetric 3D T1 and FLAIR images and high-resolution 2D submillimetric T2 images, with the idea of standardizing best-practice neuroimaging of epilepsy in outpatient clinics and specialized surgery centers alike.
Right superior frontal focal cortical dysplasia (ILAE IIb). High-resolution T2 coronal image (A) shows moderate focal cortical thickening of the right superior frontal gyrus with blurring of the gray-white matter interface (arrow). An [18F] FDG-PET scan of the brain and the fused PET/MR images (B and C) reveal focal hypometabolism corresponding to the dysplastic cortex (arrows). MEG scan (D) shows tight dipole clustering, corresponding to the site of PET and abnormal findings on MR imaging.
Large meta-analysis studies have shown that the odds of becoming seizure-free after surgery were 2.5 times higher in patients with MR imaging–identified structural lesions.7 The diagnostic accuracy is highly dependent on logistics, including image resolution, magnetic field strength, number of phased array head coils, and the expertise of the reader. Neurologists should have a low threshold for repeatng the MR imaging examination with an optimized protocol, particularly in patients with drug-resistant epilepsy and prior normal MR imaging findings, because this may reveal a lesion in 30%–65% of cases. At our institution, we use 7T as frontline imaging for all patients with suspected epilepsy and no safety contraindications. The standard epilepsy protocol uses 7T MR imaging with an 8-channel transmit and a 32-channel receive coil. An optimized 3D MP2RAGE sequence is obtained, as previously described, with the first TI giving an edge-enhancing gradient-echo (3D-EDGE) contrast with simultaneous acquisition of high-resolution T1-weighted images. We also obtain an oblique coronal high-resolution T2 TSE sequence oriented perpendicular to the hippocampus with an in-plane resolution of 300 × 300 μm and a section thickness of 1.2 mm, allowing exquisite visualization of the hippocampal subfields. High-resolution 3D FLAIR and SWI are also obtained.
Apart from other routine sequences like SWI and DWI, newer sequences like 3D-EDGE are routinely used at our center. 3D-EDGE is a recently described MR imaging sequence that offers superior contrast-to-noise in the detection of cortical malformations. The added SNR from 7T allows higher image resolution (0.8 versus 1.0 mm) while still achieving nearly twice the SNR as 3T (Fig 3).8 FDG-PET/CT/MR imaging is a well-established functional study with a detection rate of 30% and 60% for extratemporal lobe and temporal lobe epilepsies, respectively (Fig 2). A dynamic PET acquisition with a quantitative assessment is a new tool that offers higher sensitivity than static PET imaging to identify areas of hypometabolism. These advances along with developments in machine learning continue to refine our ability to detect subtle epileptogenic lesions. MR imaging findings could frequently be negative in some pathologically-confirmed FCDs (I or IIA), but this is more likely due to substandard acquisitions and the radiologist’s interpretation, often blinded to the EEG data. The proportion of MR imaging negative for FCD is expected to decline with wider availability of stronger magnets, dedicated epilepsy protocols, and highly subspecialized reads.8⇓-10
Ultra-high-field (7T) MR imaging scan with BOS FCD ILAE type IIa with a DEPDC5 mutation. Mild cortical thickening is noted at the base of left superior frontal sulcus with blurring of the gray-white matter interface and minimal T2 hyperintensity in the adjacent WM (arrows). The crown of the gyrus is normal. Newer sequences like 3D EDGE (D) can better define the gray-white interface, lost in the region of FCD, as seen here (D, arrow).
FCD
FCD is the most commonly resected epileptogenic lesion in the pediatric age group and the third most common lesion in adults. This was first identified and classified by David Taylor in 1971, on the basis of the presence of irregular dysmorphic neurons and enlarged ballooned cells in the neocortex.2,10 Since then, many classification systems have been proposed by neuropathologists and clinicians, with, however, important gaps in our knowledge about the pathogenesis and clinical characteristics of FCD. In 2004, Palmini et al10 classified FCD as “mild malformation of cortical development” (mMCD type I and II) and FCD types II and II on the basis of the neuropathologic features, with the former having heterotopic/excess neurons and FCDs having cortical dyslamination with or without dysmorphic neurons and balloon cells.11 This was one of the leading classification systems used for FCDs until 2011, when it was replaced by the Blumcke classification, which shared many features of the previous classification, with identification of the 3 subtypes of FCD and deletion of malformation of cortical development (MCD). The ILAE created a task force in 2011 to review the FCD classification and provide an update with pathologic and genetic information. A meta-analysis of published literature from 2011 identified substantial information on the EEG, clinical, and radiographic phenotypes, along with new information on genetic characterization.12
The ILAE consensus classification was published in 2022 and serves as the most updated classification scheme (Table) with widespread international agreement, and it highlights the importance of immunohistochemical staining and the phenotype-genotype integration. Major changes include the concept of the multilayered integrated diagnosis model combining radiographic and histopathologic findings with genetic information. The 4 layers in the new scheme include the histopathologic assessment (layer 1), followed by integration of molecular and genetic results (layer 2), integration of presurgical neuroimaging findings (layer 3) and the final layer of integrated diagnosis (layer 4). This new scheme highlights the role of genetics and the emerging role of epigenetics in FCD-related epilepsy. New information on the clinical, histopathologic, and genetic characteristics was most prominent in the type II category, with little changes made to types I and III. The classification identifies new entities based on histopathologic and genetic information, including FCD II located at the BOS, mMCD, MOGHE, and no definite FCD on histopathology.
| Blumcke (ILAE 2011) | ILAE 2022 |
|---|---|
| FCD type I, isolated focal cortical dysplasia | |
| Ia abnormal radial cortical lamination | Ia abundant neuronal microcolumns (vertical) |
| Ib abnormal tangential cortical lamination | Ib abnormal tangential layering |
| Ic abnormal radial and tangential cortical lamination | Ic vertical and horizontal abnormalities |
| FCD type II, isolated, focal, cortical dysplasia | |
| IIa dysmorphic neurons | IIa dysmorphic neurons |
| IIb dysmorphic neurons and balloon cells (Taylor type) | IIb dysmorphic neurons and balloon cells (Taylor type) |
| BOCa FCD (new entity) could be IIa or IIb on histopathology) | |
| FCD type III, cortical dyslamination and principal lesion | |
| IIIa with hippocampal sclerosis | IIIa with hippocampal sclerosis |
| IIIb adjacent to glial/glioneuronal tumor | IIIb adjacent to glial/glioneuronal tumor |
| IIIc adjacent to vascular malformation | IIIc adjacent to vascular malformation |
| IIId adjacent to early life insult like ischemia | IIId adjacent to early-life insult-like ischemia |
| Term of “not otherwise specified (NOS)” should be used if microscopic diagnosis is not based on appropriate immunohistochemical staining, eg, FCD type II (NOS) | New entities |
| mMCD increase in heterotopic neurons in the WM | |
| MOGHE indicates mild malformations of cortical development with oligodendroglial hyperplasia (>2200 Olig-2 cells/mm) | |
| No definite FCD on histopathology, ambiguous pathologic findings, not compatible to FCD I or II |
↵a Common immunohistochemical staining used for FCD diagnosis includes antibodies directed against NeuN neurofilaments, vimentin, MAP2, CD34, OLIG2, glial fibrillary acid protein, or α B-crystallin.
Comparison of Blumcke 2011 classification of FCDs with the updated ILAE 2022 classification, along with the new 2022 entities
The histopathologic classification is best achieved by immunohistochemical stains such as NeuN, which is most helpful in deciphering the 6-layered architecture of the neocortex (Fig 4A). Multiple FCD subtypes can exist together, and different severity grades can be seen in the histopathologic sample of same FCD type, suggesting a common molecular mechanism with a spectrum of appearances. Given the numerous iterations and revisions of various classification during the past 2 decades and the notable overlap among the schemes, it would be best practice to explicitly state which classification system is being used.2,13 Globally, the Blumcke classification has the widest acceptance at present; however, it would be prudent for the radiologist to use the ILAE classification in their reports and refrain from use of obsolete terminology like “Taylor type FCD.”
NeuN immunohistochemistry highlighting the neuronal cell in the normal cortex and FCDs I–III. Normal 6-layer architecture is depicted in the normal cortex (A). FCD Ia (B) shows abundant neuronal microcolumns. Low-power magnification of the cortex in FCD IIb shows complete loss of layering and many enlarged, clustered dysmorphic neurons (C, black arrows). Balloon cells are not clearly delineated on NeuN immunohistochemistry and are better seen on stains like crystallin (see Fig 5D). FCD IIId in a young adult with a remote ischemic insult reveals cortical thinning with marked loss of neurons in the middle layer 4 (D, arrow).
Although the new ILAE classification scheme offers standardization across different clinical specialties and geographic areas, many challenges and limitations exist. First, there is wide variability in the availability of resources, including access to high-field (3T or 7T) MR imaging and advanced neuropathology and genetic labs.2 Second, the ILAE histopathologic assessment emphasizes en bloc resection using anatomic landmarks, which is not feasible in many cases. Surgical procedures resulting in piecemeal fragmentations of tissue can cause errors in the pathologic classification. Furthermore, nonsurgical techniques like laser ablation, thermocoagulation, and neurosurgical sampling errors can restrict tissue availability.7 Low interobserver agreement on the histopathologic classification, especially, occurring in the assessment of type I and III FCDs, can also limit accurate classification. The concept of integrated diagnosis in the latest ILAE classification aims to counteract some of these limitations with more focus on the genomic information like the mechanistic target of rapamycin (mTOR) pathway and mutations of tuberous sclerosis (TSC) gene. It is expected that the future classification will be more objective and molecular-based, similar to the new World Health Classification CNS Tumor.14
FCDs can be in any part of the cortex, can be variable in size and location, and can involve multiple lobes. Type II FCDs are more commonly noted in the extratemporal lobes, usually in the frontal region. The main clinical presentation of FCDs is epilepsy (often drug-resistant), which can start at any age, with high seizure frequency noted in type I and II lesions. The age of epilepsy onset can range from younger than 1 year to 60 years of age, with epilepsy starting before the age of 5 years in most (61%) and before the age of 16 years in >90% of patients.15 An early age of onset is observed in type I FCDs compared with other types. In patients with mMCD, the age of epilepsy onset is homogeneously distributed across the first 2 decades. The association of the behavioral and emotional component is increasingly being recognized and is emphasized in the new classification. The characteristic pattern on EEG includes focal, rhythmic epileptiform discharges, often showing spatial correlation with the lesion. There has been continuous improvement in clinical outcomes after surgery with advancement of preoperative diagnostic methods and surgical techniques. The results are dependent on several factors, including pathologic etiologies, extent of the lesion, and types of surgery, with the overall seizure-free outcome after surgery ranging from 50% to 75%.14 Complete resection is widely accepted to be the most important prognostic factor for surgical outcome. Better clinical outcome has also been observed in patients with demonstrated MR imaging lesions and in temporal lobe resections. Some studies have shown better rates and higher seizure freedom with type II and III FCDs compared with type I, though this finding has not been firmly established.7
FCD I
FCD type I has specific histopathologic features with architectural disorganization of the neocortex secondary to altered developmental maturation, without evidence of any additional principal epileptogenic lesion in the brain (on imaging or pathology). No changes have been made to this category from the 2011 (Blumcke) classification.12 This issue is best evaluated on NeuN staining with type Ia having an abundance of neuronal “microcolumns,” abnormal radial migration, and an excess of heterotopic neurons in the adjacent white matter (Fig 4B). Type Ib has abnormal layering (tangential), whereas type 1c has both horizontal and vertical abnormalities. The presence of dysmorphic neurons or “balloon cells” on histopathology excludes this diagnosis. Type I and III categories remain difficult to distinguish from the normal variation in cortical architecture, more so if in the temporal lobes. FCD type I associated with any primary or principal lesion like hippocampal sclerosis or glioneural tumors is considered FCD type III. No strong genetic correlation has been established yet; however, this may change in the future, and new types could be recognized.2,13 FCD type I is challenging to diagnose on imaging, with the lowest detection rate of all FCD types and a very high percentage (50%) of MR images read as having normal findings.16 The widespread opinion that MR imaging usually has negative findings in FCD I is gradually changing with greater expertise and high-resolution ultra-high-field MR imaging. FCD I is typically seen as blurring of the gray-white matter interface (though less pronounced compared with type II) with prominent segmental or lobar atrophy, loss of regional WM volume, and moderately increased T2 FLAIR hyperintensity in the adjacent WM. These findings are usually confined to the temporal lobes (Ia > Ib) and are less commonly seen in frontal region.16,17
FCD II
FCD II is a highly epileptogenic lesion that is commonly associated with early-onset drug-resistant focal epilepsy and is the most common cortical dysplasia in epilepsy surgery. Although no changes were made to the classification scheme, there were significant changes to the genetic pathways identified in this category. There was identification of a special BOS variant along with recognition of specific genetic mutations in this category. The pathologic hallmark of this group includes cortical dyslamination and dysmorphic neurons without (IIa) or with balloon cells (Fig 4 C, -D). Balloon cells are immature cellular entities with a large cell body that lacks Nissl substance, closely resembling giant cells seen in TSC. Numerous genetic variants have been identified in FCD II, including single hit (ie, gain-of-function variant) inactivators of the mTOR pathway or the better-established double-hit with a germline and somatic loss-of-function variant (ie, DEPDC5, NPRL2, NPRL3, TSC) pathway.2 FCD II more commonly involves the frontal lobes, with imaging changes including abnormal gyration patterns indicated by a cortical dimple, cortical thickening, and blurring of the gray-white matter interface. Cortical thickening is significantly more pronounced in the type II FCDs compared with types I and III. Increased T2 signal within the dysplastic cortex and the adjacent WM is frequently seen. Despite an increase in T2 signal, the cortex remains hypointense to the much brighter adjacent WM. The transmantle sign refers to a linear or triangular band of T2 FLAIR WM hyperintensity extending from the lesion toward the ventricle, usually typical of FCD IIb seen in >80% of patients with IIb FCD, primarily due to the reduction of myelinated fibers (Fig 5). WM signal changes, per se, cannot distinguish FCD II from non-FCD II subtypes; however, the tapering pattern (transmantle sign) has high specificity for FCD II (IIb > IIa). New imaging markers have been described in FCD IIb using 7T magnets and SWI sequences, including an intracortical band of hypointense signal, termed the “black line,” located in the deep layers or cortex, representing abnormal iron deposition. This is the subtype most commonly identified on repeat MR imaging using 7T scanning instead of 3T.16,18
Right frontal ILAE type IIb in a 30-year-old patient with focal partial symptomatic epilepsy and epileptic syndrome. Coronal 3D EDGE (A) and T1-gradient-echo image (MP2RAGE) (B) obtained on a 7T magnet reveal cortical thickening with blurring of gray-white matter interface (arrows). Note a transmantle band reaching up to the ventricle margins, best seen on the T2 FLAIR coronal image (C, arrow). MP2RAGE depicts the thickening and blurring of the cortex at the site of the FCD with sharp gray-white demarcation at the normal cortices, showing the superiority of T1-weighted gradient sequences for anatomic delineation. Multiple balloon cells in FCD IIb on crystallin staining of the dysplastic cortex are seen as large, round cells with oval, eccentric nuclei; prominent nucleoli; and abundant cytoplasm scattered throughout the GM (D, black arrows).
BOS FCD.
BOS FCD is a relatively recently identified intrinsically and focally epileptogenic lesion, similar to type IIb (more common) or type IIa on pathology. These lesions are restricted in their anatomic location and extent to the BOS, best delineated on MR imaging with a high rate (>90%) of surgical cure.19 These are most frequently seen in the frontal lobe sulci (superior frontal sulcus, inferior frontal sulcus, and central sulcus) with a characteristic rhythmic spiking pattern in the depth of the sulcus lesion on EEG. Imaging findings are similar to those of any FCD II, with, however, better anatomic demarcation between the dysplastic and normal cortex, with complete cure on total resection of the lesion. From a histopathologic standpoint, the lesions show cellular and architectural patterns of either FCD IIb (Fig 3) or, less commonly, FCD IIa. The most common genetic mutation in this entity involves the mTOR pathway (“mTORpathies”) or the DEPDC5 double-hit variant. This is an imaging entity in which the neuroradiologist plays a vital role in the diagnosis, offering a high chance of complete cure.2,20
FCD III
FCD III is similar histopathologically to type I FCDs and refers to cortical lamination abnormalities associated with a principal lesion, usually adjacent to or affecting the same cortical region. Dysmorphic neurons seen in type II are not a feature of FCD III and exclude the diagnosis of either FCD I or III. There are 4 variants included in this category, including the following: IIIa associated with hippocampal sclerosis; IIIb associated with tumors (Fig 6); IIIc associated with vascular malformations; and IIId associated with lesions acquired during early life. The latter (IIId) comprises a large spectrum of lesions secondary to traumatic brain injury, glial scarring after ischemic injury, and inflammatory or infectious diseases such as Rasmussen encephalitis. This category has been largely preserved from the 2011 Blumcke classification with no significant genetic association identified. The imaging features are dominated by the principal lesion rather than the cortical dysplasia. Surgical outcome depends on the principal/primary pathology and is independent of the coexisting FCD.2,12
Left frontal ILAE type IIIb in an 18-year-old patient in conjunction with multinodular and vacuolating neuronal tumor (MVNT). A cluster of multinodular T1-hypointense and T2 FLAIR-hyperintense nodules (A, B, and D, white arrows) is seen in the left posterior peri-Sylvian region (supra-Sylvian perirolandic and posterior insula) consistent with MVNT. Note a dysplastic adjacent cortex involving the inferior frontal gyrus (C, black arrow) with cortical thickening and blurring of the gray-white matter interface.
Newly Recognized Entities
Three new entities included in the ILAE 2022 classification are mild mMCDs, MOGHE, and no definite FCD on histopathology. The 2004 classification of Palmini et al10 included MCD with the observation of excess heterotrophic neurons in the cortical layer 1 and the WM, classified as subtype I and II MCDs, respectively. The subtype I MCD with hypertrophic neurons in the cortical layer was, however, not confirmed in the surgical cases, resulting in deletion of this entity. mMCD in the 2022 ILAE classification is more objectively defined as an excess of heterotopic neurons in the WM with >30 neurons per square millimeter. This category has been associated with mutations of the sodium‐activated potassium channel gene KCNT1 and sleep‐related hypermotor epilepsy. Large surgical series have reported this feature in around 3% of patients with epilepsy, with most studies reporting normal imaging findings.2
Mild MOGHE is a new ILAE 2022 entity, defined by an increase in heterotopic neurons in the WM and oligodendroglial cell densities above 2200 Olig2-immunoreactive cells per millimeter, along with an increased neuropil count of the WM (>10%). This description usually involves young children with a median age of seizure onset of 2 years, usually presenting with frontal lobe or, less frequently, temporal lobe epilepsy. This can be a subtype of mMCD with distinctive histopathologic features and a strong association with SLC35A2 genetic mutations (45%–100%). Although MOGHE is primarily a WM abnormality, it can be associated with abnormal cortical folding on MR imaging. Excellent clinical outcome has been seen in this category with large surgical resections.2,21 There is a paucity of literature on the imaging findings in MOGHE, with a few early studies revealing subtle cortical thickening and blurring of the GM matter junction and an alteration of adjacent WM myelination pattern (Fig 7).
MOGHE in a 2-year-old boy with surgical pathology confirmation. Multiple T2 FLAIR images (A–C) reveal focal subcortical WM hyperintensity in the right middle frontal gyrus region (arrows). Coronal T1 gradient-echo image (MPRAGE) (D) shows a normal overlying cortex with subtle hypointensity (arrow) corresponding to the WM signal changes seen on T2/FLAIR. A right frontal resection was performed with excellent seizure control. Histopathology revealed subcortical WM hypercellularity, largely oligodendroglial (on Olig-2 stains), suggestive of oligodendroglial hyperplasia. NeuN highlighted a few scattered neurons in the WM; however, no dysmorphic neurons, balloon cells, or cortical dysplasia was noted.
Recent studies have shown that MR imaging findings of MOGHE are age-related, with different features in adults versus children. Findings include an increased laminar T2 and FLAIR signal at the corticomedullary junction in young children (subtype I), with poor corticomedullary differentiation due to increased T2 signal of the adjacent WM in older patients (subtype II).22 Lesions are subtle, and the presence of a cortical dimple, best seen on T2-weighted images, can greatly help in localization.22,23 Finally, a new category of no definite FCD on histopathology was introduced, which applies to ambiguous neuropathologic results and does not fit in any of the above histopathologic categories. There is no literature specifically describing the imaging findings in this last category, at present.2
CONCLUSIONS
Integration of genetic information into the clinical-imaging phenotype is becoming more common across the board from oncology to autoimmune conditions, with wider availability of dedicated gene panels. The 2022 ILAE classification of FCD is an effort to incorporate genetic information with histopathology and clinical and radiographic information with the idea of providing a multilayered integrated diagnosis, like the classification of brain tumors. This latest classification is also an effort to reduce the ambiguity due to different classification schemes that have developed in parallel with numerous iterations and revisions. Finally, it is expected that the new classification scheme will foster better multidisciplinary research with easier modifications as more genetic information becomes available in the coming decade.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received July 26, 2023.
- Accepted after revision December 13, 2023.
- © 2024 by American Journal of Neuroradiology