Abstract
BACKGROUND AND PURPOSE: The radiologic evaluation of ongoing myelination is currently limited prenatally. Novel quantitative MR imaging modalities provide relaxometric properties that are linked to myelinogenesis. In this retrospective postmortem imaging study, the capability of Synthetic MR imaging and MR fingerprinting–derived relaxometry for tracking fetal myelin development was investigated. Moreover, the consistency of results for both MR approaches was analyzed.
MATERIALS AND METHODS: In 26 cases, quantitative postmortem fetal brain MR data were available (gestational age range, 15 + 1 to 32 + 1; female/male ratio, 14/12). Relaxometric measurements (T1-/T2-relexation times) were determined in the medulla oblongata and the midbrain using Synthetic MR imaging/MR fingerprinting–specific postprocessing procedures (Synthetic MR imaging and MR Robust Quantitative Tool for MR fingerprinting). The Pearson correlations were applied to detect relationships between T1-relaxation times/T2-relaxation times metrics and gestational age at MR imaging. Intraclass correlation coefficients were calculated to assess the consistency of the results provided by both modalities.
RESULTS: Both modalities provided quantitative data that revealed negative correlations with gestational age at MR imaging: Synthetic MR imaging–derived relaxation times (medulla oblongata [r = −0.459; P = .021]; midbrain [r = −0.413; P = .040]), T2-relaxation times (medulla oblongata [r = −0.625; P < .001]; midbrain [r = −0.571; P = .003]), and MR fingerprinting–derived T1-relaxation times (medulla oblongata [r = −0.433; P = .035]; midbrain [r = −0.386; P = .062]), and T2-relaxation times (medulla oblongata [r =−0.883; P < .001]; midbrain [r = −0.890; P < .001]).The intraclass correlation coefficient analysis for result consistency between both MR approaches ranged between 0.661 (95% CI, 0.351–0.841) (T2-relaxation times: medulla oblongata) and 0.920 (95% CI, 0.82–0.965) (T1-relaxation times: midbrain).
CONCLUSIONS: There is a good-to-excellent consistency between postmortem Synthetic MR imaging and MR fingerprinting myelin quantifications in fetal brains older than 15 + 1 gestational age. The strong correlations between quantitative myelin metrics and gestational age indicate the potential of quantitative MR imaging to identify delayed or abnormal states of myelination at prenatal stages of cerebral development.
ABBREVIATIONS:
- GA
- gestational age
- ICC
- intraclass correlation coefficient
- MBP
- myelin basic protein
- MDME
- multidynamic multiecho
- MRF
- MR fingerprinting
- SSFP
- steady-state free precession
- T1R
- T1-relaxation times
- T2R
- T2-relaxation times
SUMMARY
PREVIOUS LITERATURE:
Relaxometric MR imaging – such as the Synthetic MR imaging- and MR Fingerprinting (MRF)-based method – represents a novel imaging approach with which to quantify ongoing myelination processes noninvasively by using various metrics [ie, T1-/T2-relexation times (T1R/T2R)]. Previous investigations have proved the value of quantitative imaging for tracking myelinogenesis even at the earliest maturational stages postnatally. However, while the process of myelination initiates prenatally, imaging studies for mapping biochemical aspects of fetal brain development are still scarce. Furthermore, there is a lack of information about consistency of results obtained by different quantitative MR modalities.
KEY FINDINGS:
Quantitative, Synthetic MR imaging- and MRF-derived T1R and T2R metrics represent sensitive markers with which to characterize myelin-related changes of the brainstem throughout the course of antenatal development. Both investigated imaging techniques provide consistent results for tracking ongoing myelinogenesis.
KNOWLEDGE ADVANCEMENT:
Despite current limitations for its in vivo application, this postmortem study indicates that relaxometric MR modalities provide novel possibilities in assessing biochemical aspects of human fetal brain maturation. Based on a multi-parametric fashion, these techniques provide the potential to identify delayed or abnormal states of myelination prenatally.
Myelination processes represent a complex aspect of brain maturation, which is highly vulnerable to external confounders, such as prenatal stress.1,2 At early developmental stages, the assessability of ongoing myelinogenesis is limited with conventional MR imaging data, due to the low sensitivity of standard-of-reference contrasts to the deposition of small myelin quantities.1,3 Thus, while the evaluation of the individual myelination state, and, therefore, the imaging-based diagnosis of respective disorders, succeeds at more advanced developmental stages (ie, after the second year of life is completed), conventionally acquired MR images currently show poor performance regarding monitoring of the biochemical aspects of cerebral maturation perinatally.1,3,4
Novel quantitative imaging approaches have already proved beneficial to increase the sensitivities of MR imaging for tracking myelin deposition even at the earliest stages of cerebral development.5 Relaxometric imaging data, such as Synthetic MR Imaging and MR fingerprinting (MRF), hold the potential to provide multiple contrasts based on a single scan.3,5,6 Both modalities use a single sequence (multidynamic multiecho [MDME]-based [Synthetic MR imaging] versus steady-state free precession ([SSFP]-based [MRF] acquisitions), which allows reconstruction of various imaging data via retrospective postprocessing modulations.3,6 Moreover, these techniques allow retrieval of metric information (ie, T1- and T2-relaxation times [T1R/T2R]), which are considered directly linked to ongoing myelin deposition.5⇓-7
While quantitative MR modalities have been studied extensively in the neonatal period, descriptions that focus on their applicability prenatally are still scarce.3,5,7,8 In addition, studies that compare the consistency of the results determined by different quantitative techniques are currently lacking. Quantitative MR imaging may hold the potential to characterize delayed or abnormal stages of myelination and, thus, open new possibilities in the individual assessment of different aspects of brain maturity prenatally.3,5
The aims of this investigation were the following: 1) to study myelin development in the fetal period using a quantitative MR-based approach, and 2) to prove the consistency of the results provided by 2 different quantitative MR modalities. Thus, postmortem Synthetic MR imaging and MRF-based imaging were performed in fetuses following medically indicated termination of pregnancy. Because myelinogenesis proceeds in a caudal-to-rostral fashion throughout perinatal development, myelin deposition was quantified in the fetal brainstem.1,9 The results of both modalities were compared and correlated against the individual developmental stages (ie, gestational age [GA]) at the time of MR imaging.
MATERIALS AND METHODS
Ethical Approval
The protocol of this retrospective study was reviewed and approved by the review board/ethics committee of the Medical University of Vienna (institutional review board approval number: 1585/2023). The described procedures were performed in accordance with the Declaration of Helsinki. All guardians were informed about postmortem MR imaging and agreed to the scientific use of the acquired imaging data.
Study Cohort
At our institution, postmortem imaging is performed routinely in fetuses who underwent MR imaging in utero and in vivo before the medically indicated termination of pregnancy (either by fetocide or drug-induced using mifepristone), due to diagnostic quality assurance–related issues. Between January 2021 and March 2023, a total of 29 fetal postmortem MR examinations were performed. MR imaging was performed at the Department of Neuroradiology after referral for postmortem MR imaging by the Department of Obstetrics and Gynecology of the same institution. The Online Supplemental Data give an overview of the fetuses included. Postmortem fetal MR data were included only when axial acquisitions were available. Fetuses imaged before 15 weeks +0 days GA were excluded, due to coil- and specimen size-related artifacts (ie, signal voids, image quality degradation, and so forth).
Postmortem MR Imaging and Postprocessing
Postmortem MR imaging was performed between 6 and 96 hours after medically indicated termination of pregnancy. In the time span between the termination of pregnancy and imaging, the fetal specimens were kept well-preserved in a refrigerator at 4°C. Postmortem MR imaging was performed on one 3T MR system (Magnetom Vida; Siemens) equipped with a 64-channel receiver coil. All fetuses were examined using a standardized postmortem MR imaging protocol (Online Supplemental Data). To obtain quantitative imaging data, we acquired MDME (Synthetic MR imaging) and SSFP (MRF) sequences (Table 1). MR data-postprocessing was performed using SyMRI (Version 11.2; SyntheticMR AB) (MDME sequences) and the syngo.via-based tool MR Robust Quantitative Tool (MR-RoQT, Version 2.0.11; Siemens) (SSFP sequences) to determine quantitative imaging metrics.
Quantitative sequence characteristics
Determination of Quantitative Properties
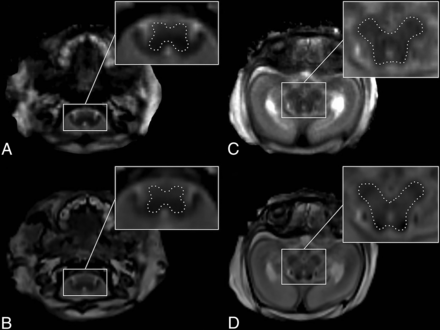
As described in previous (prenatal and postnatal) quantitative MR imaging studies,5,7 the T1R (millisecond) and T2R (millisecond) metrics were measured in ROIs in the brainstem that are considered to hold the highest quantities of intracranial myelin prenatally: the medulla oblongata (level of the inferior olive) and the midbrain (level of the quadrigeminal plate).1,7,9 ROI placement was performed manually (ie, manual delineation of the individual contours of the medulla oblongata and the midbrain) on Synthetic MR imaging and MRF-based maps (generated by SyMRI and MR-RoQT, respectively) (axial plane) (Fig 1) by 2 neuroradiologists with at least 2 years of experience with fetal MR imaging. The obtained quantitative T1R/T2R metrics represent an average value (based on each voxel within the drawn ROI), which was used for further analysis.
ROI placement is demonstrated in a female fetus (postmortem MR imaging performed 68 hours after medically indicated termination of pregnancy due to corpus callosum agenesis with cortical malformations at 28 + 0 GA) on T2R-based maps provided by MRF (A and C) and Synthetic MR imaging (B and D). A and B, ROI drawings of the medulla oblongata. C and D, ROI drawings of the midbrain.
Statistical Analyses
Statistical computations were performed using SPSS Statistics for Macintosh (Version 27.0; IBM) as proposed by a biomedical statistician with 10 years of experience in the field. The significance level was set at α = 5% (P < .05).
To detect relationships between the developmental stages (ie, fetal GA) at the time of postmortem MR imaging and the individual myelination state (as deduced on the basis of the quantitative MR metrics), Pearson correlation analyses were performed between T1R/T2R (as provided by Synthetic MR imaging and MRF, respectively) and GA.
To compare the consistency of the results (ie, T1R/T2R metrics) based on both quantitative imaging modalities, we calculated an intraclass correlation coefficient (ICC) (2-way mixed for consistency). Moreover, another ICC analysis (2-way mixed for absolute agreement) was performed to prove the repeatability of the manually determined, quantitative measures obtained by both observers. The ICC results were interpreted as proposed by Cicchetti:10 <0.4, poor; 0.4 to 0.6, fair; 0.6 to 0.75, good; and >0.75, excellent.
RESULTS
Twenty-six/29 cases (mean GA [weeks + days] at MR imaging: 23 + 2 [±5 days] [range, 15 + 1 – 32 + 1]; female/male ratio: 14/12]) (Online Supplemental Data) were included in this study. MDME sequences (Synthetic MR imaging) were available in 25/26 postmortem MR acquisitions. There were 24/26 SSFP scans (MRF) included in this study. In 23/26 fetuses, both Synthetic MR imaging and MRF-based data were provided. Figure 2 gives an overview of included and excluded cases.
The flow diagram indicates how the study sample was derived. Overall, 29 postmortem fetal MR data sets were acquired between January 2021 and March 2023. Only axial postmortem fetal MR data acquired at >15 + 0 (weeks + days) GA were included (n = 26). Cases in which no axial imaging data were available (n = 1) and those data that were acquired <15 + 0 GA (n = 2) were excluded from this study.
Relationships between Myelin-Related Metrics and Developmental Stages
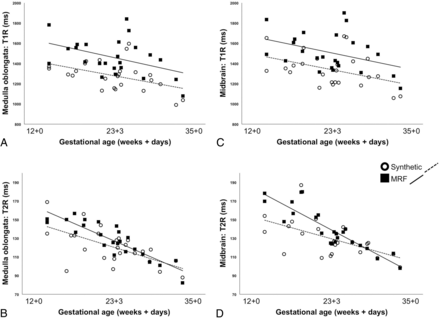
For synthetic MR-based data, there were significant correlations between the GA at the time of postmortem MR imaging and the T1R (determined in the medulla oblongata, r = −0.459; P = .021; and the midbrain, r = −0.413; P = .040) and the T2R (determined in the medulla oblongata, r = −0.625; P < .001; and the midbrain, r = −0.571; P = .003).
For MRF-based data, there were significant correlations between the GA at the time of postmortem MR imaging and the T1R (determined in the medulla oblongata, r = −0.433; P = .035), and the T2R (determined in the medulla oblongata, r = −0.883; P < .001, and the midbrain, r = −0.890; P < .001). There were no significant correlations between the GA at the time of postmortem MR imaging and the MRF-derived T1R (determined in the midbrain, r = −0.386; P = .062) (Figs 3–5).
Demonstration of the relationships between Synthetic MR imaging and MRF-based T1R (A and C) and T2R (B and D) metrics (determined in the medulla oblongata [A and B] and the midbrain [C and D]) and the individual developmental stages at the time of postmortem fetal imaging.
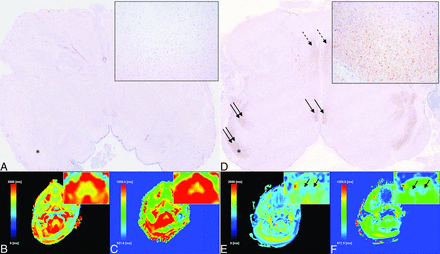
Correlation between histology and MR imaging (medulla oblongata: level of the inferior olivary nucleus) at different developmental stages (24 + 1 [A, B, and C] [termination of pregnancy due to congenital disorder of glycosylation syndrome] versus 32 + 1 [D, E, and F] [termination of pregnancy due to Coffin-Siris syndrome]) based on immunohistochemical detection of MBP (A and D), SyMRI (B and E), and MRF-derived (C and F) quantitative T1R-mapping (ie, color-coded voxels according to the respective T1R metrics [as indicated by the color scale in B, C, E, and F]). The MBP-based histology specimens (A [magnification: ×2.1] and D [magnification: ×1.3]) reveal considerable myelination (brown staining) (magnification [×100] [right upper image elements in A and D of myelin deposited within the inferior cerebellar peduncle [asterisks in A and D]) in the dorsal/tegmental brainstem (inferior cerebellar peduncle [double arrows in D] and medial longitudinal fascicle [arrows in D]) and the medial lemniscus (dotted arrows in D) at 32 + 1, while myelin is relatively scarce at 24 + 1. Quantitative SyMRI (B and E) and MRF-based (C and F) imaging demonstrates myelin-related relaxation time shortening (bluish color-coding) in the dorsal medulla oblongata (arrows in E and F) (foremost induced by myelin deposited within the inferior cerebellar peduncles) at 32 + 1, which is absent at 24 + 1. These MR-based findings are in good agreement with histology and underline the high sensitivity of quantitative imaging for early stages of myelin development.
Comparison between histology (postmortem at 30 + 0 GA) (termination of pregnancy due to agenesis of the corpus callosum and suspected Coffin-Siris syndrome) and nearly age-matched postmortem MR imaging in another case (MR imaging at 31 + 2 GA) (termination of pregnancy due to tubulinopathy). Klüver-Barrera staining (A and C) and immunohistochemical detection of MBP (B and D) of the medulla oblongata (level of the inferior olivary nucleus) (A and B) and the midpons (C and D) are shown. T2 BLADE (reference MR image) (E) and SyMRI (F) versus MRF-based (G) quantitative T1R-mapping (ie, color-coded voxels according to the respective T1R metrics as indicated by the color scale in F and G) is demonstrated. While Klüver-Barrera staining of the medulla oblongata (A) and the pons (C) does not reveal myelinated tissue (myelin would appear as a bluish staining), MBP-based histology already depicts myelination (brown staining) of the medial lemniscus (asterisks) (longest diameter: 1.6 mm), the lateral lemniscus (arrowheads) (longest diameter: 0.7 mm), the medial longitudinal fascicle (arrows in B and D) (longest diameter: 0.8 mm), and the inferior cerebellar peduncles (dashed arrows), which is accompanied by a subtle brownish background staining within the dorsal or tegmental areas, respectively. As opposed to other myelin-staining methods (eg, Klüver-Barrera), MBP-based histology is considered more sensitive to early stages of myelination.27 Quantitative SyMRI (F) and MRF-based (G) imaging already demonstrates relaxation time shortening (bluish color-coding) in the dorsal/tegmental brainstem at the pontomedullary junction (arrows in F and G), which is in good agreement with MBP-based myelin staining and underlines its sensitivity for “premyelination” states.24
Consistency of Synthetic MR Imaging and MRF-Derived Quantitative Imaging Metrics
The ICC between both quantitative imaging modalities ranged between good (medulla oblongata [T2R]: 0.661; 95% CI, 0.351–0.841) and excellent (midbrain [T1R]: 0.920; 95% CI, 0.821–0.965) consistency (Table 2).
Consistency between synthetic MR imaging and MRF
Interrater Reliability
The ICC between both raters for Synthetic MR imaging and MRF-derived quantitative imaging metrics revealed excellent agreement (range between: midbrain [Synthetic MR imaging-based T2R] 0.909; 95% CI, 0.805–0.959 and the medulla oblongata [MRF-based T1R] 0.981; 95% CI, 0.957–0.992) (Online Supplemental Data).
DISCUSSION
This investigation compared 2 different quantitative imaging modalities on the basis of postmortem fetal MR data. Moreover, the relaxometric properties obtained by both imaging techniques were used to study ongoing myelination processes throughout antenatal development. The quantitative results provided by Synthetic MR imaging and MRF revealed good-to-excellent consistency. Furthermore, both MR approaches revealed the feasibility of tracking proceeding myelinogenesis in the fetal period. Thus, this study proves the value of quantitative imaging to obtain insight into the biochemical aspects of brain maturation noninvasively, which are currently difficult to uncover by means of standard-of-reference MR imaging acquisition principles and conventional radiologic evaluation strategies.3,6,7,11
Myelin is first detectable in the column of Burdach at the beginning of the second trimester and progresses cephalad.1,9,12 Biochemical changes in myelin composition throughout cerebral development lead to alterations of the physical properties of the brain tissue and, therefore, to white matter signal intensity characteristics on MRI.1 However, although T1-weighted pulse sequences represent the current criterion standard with which to evaluate ongoing myelin deposition perinatally, the relaxometric changes induced by only small myelin quantities antenatally are too subtle to cause visually perceivable signal intensity changes. This issue currently hinders reliable qualitative assessments of ongoing myelination in fetal imaging settings.1,13,14 Thus, the direct retrieval of tissue-specific relaxometric properties appears to represent a viable alternative to qualitative evaluations to render tracking of myelin development feasible, even within an antenatal MR-based diagnostic framework.6,7,15 In accordance with prior descriptions in the literature that focused on quantitative MR imaging in vivo and due to the relative predominance of myelinated tissue infratentorially at early developmental stages, ROI placement for relaxometric analysis was performed in the developing medulla oblongata and the midbrain.1,5,7,9,12 Synthetic MR imaging and MRF represent the current state-of-the-art in terms of quantitative imaging.6,11 While both techniques provide tissue-specific relaxation properties, their approach to data acquisition is of a different nature.6,11 As opposed to Synthetic MR imaging, in which a spin-echo-based acquisition principle is used, MRF operates via a signal-recognition process to determine relaxometric features on the basis of a gradient-echo sequence.6,16⇓⇓-19 Therefore, while T1R/T2R are measured directly in Synthetic MR imaging (ie, T2R: obtained via a multiecho technique; T1R: obtained via the application of various 90° radiofrequency pulses at different saturation recovery times), MRF retrieves these metrics via a database with which the obtained signals are matched (ie, SSFP sequences induce evolutions of tissue-specific signals that are then allocated to certain physical/MR-based properties).6,11,16⇓⇓-19
There was considerable consistency between both techniques. These results appear credible, because relaxometric properties are considered relatively robust.20 There is evidence that the accuracy for T1R is slightly higher than that of T2R, which is in line with the presented data.20 This feature may be attributed to potential biases caused by magnetic field inhomogeneity that are considered more pronounced in gradient-echo-based sequence acquisitions and T2R metrics.21 Additionally, the impact of temperature on tissue-specific relaxation properties has considerable implications on this study.22 As described by Petrén-Mallmin et al,22 reductions in temperature are associated with decreasing T1R/T2R metrics. Thus, because postmortem MR imaging mandates the need for specimen cooling before imaging, its effects must be considered when interpreting the presented data. However, minor deviations between techniques are in keeping with previous investigations.18
Both modalities revealed relationships between relaxometric properties and individual developmental stages, with more advanced GA associated with decreases in T1R and T2R. These findings are in line with various descriptions in the literature.7,23 In a previous fetal imaging study, MDME-based relaxometry was applied in vivo.7 Even though this prior study was performed at 1.5T in fetuses older than 23 weeks’ GA, the observed decreases in quantitative MR parameters are comparable with those reported in the presented investigation.7 Moreover, similar findings were detected in former preterm neonates, imaged at various stages of postnatal development.5 The T1R is believed to have sensitivity even for “premyelination” states.5 Before actual fiber ensheathment, myelin building blocks, such as cholesterol, proteins, and glycolipids interact with H2O (ie, “premyelination” stage).5,24 Concomitantly, the overall water content of the tissue certainly decreases, resulting in shortening of the T1R.5,24 With time, there is winding of loose myelin membranes around the neural axons, accompanied by progressive entry of organic compounds into the sheath, further decreasing the T1R and the overall proton density.1,5,24 As opposed to the T1R, changes of the T2R appear considerably later in the course of myelinogenesis.1,24 Following development of the bilayer, there is tightening of closely packed wraps of relatively mature myelin sheaths, which causes the T2R to decrease.1,24 Therefore, detailed observations of quantitative parameter shortening appear to allow approximations of the actual state of myelination and myelin content, respectively, as demonstrated and confirmed by Figs 4 and 5.
On the basis of these considerations, Synthetic MR imaging may be slightly more sensitive than MRF in the early developmental stages, because the latter did not reveal any significant relationships between T1R in the midbrain and GA. Nonetheless, sample size–related issues and variabilities of postprocessing algorithms could also explain these minor differences between techniques.20,25 Most interesting, there is evidence that the T2R properties may allow a more sophisticated distinction between various states of myelin maturation (ie, completely or incompletely myelinated and unmyelinated tissue), which may explain the highly significant correlations between developmental stages and T2R1. Thus, overall, both investigated approaches proved feasible for capturing the complexities of biochemical brain maturation on an objective, quantitative basis. Moreover, in this study, an interrater reliability analysis has proved the reproducibility of the quantitative, manually determined measurements and, therefore, the robustness of both techniques.
Both investigated techniques use a single-sequence-based approach with which to derive multiple MR contrasts and relaxometric properties to uncover microstructural aspects of the investigated tissue of interest.3,5,6 These benefits are of greatest interest for prenatal diagnostics. Nonetheless, sequence acquisition–related drawbacks currently limit the feasibility and application of fetal quantitative MR imaging in a clinical in vivo setting, mandating the need for further optimization (ie, k-space sampling, acceleration techniques, and so forth).7 Therefore, this study focused on postmortem MR data to further expand the knowledge of the potential of relaxometric prenatal MR imaging, despite its current limitations. However, technical advances (eg, single sequence–derived 3D-based MR relaxometry) are progressing rapidly and may soon render quantitative MR imaging feasible in clinical fetal imaging practice.26
Certain limitations require consideration. There were slight differences in acquisition parameters for both modalities, which prohibited the investigation of absolute agreement between the quantitative metrics obtained by Synthetic MR imaging and MRF. Nevertheless, despite certain differences in metric data, Synthetic MR imaging and MRF provided comparable results, because the consistency between both techniques was highly satisfactorily, particularly when considering certain frame conditions associated with postmortem imaging and quantitative MR imaging (eg, variability of delays between termination of pregnancy and MR imaging and the accompanied variance in postmortem edema at the time of imaging, as well as computational variability in postprocessing procedures, and so forth).20,22 As a matter of course, medically indicated termination of pregnancy was performed only in fetuses who revealed serious pathology. Thus, certain conditions may have affected myelin development and, therefore, the presented data do not represent reference values for regular antenatal myelin maturation. Nonetheless, the investigated modalities have the potential to provide such data, which should be further elaborated in the future.
While this study focused on the midbrain and the medulla oblongata, the pontine region was not considered for this investigation, due to its relatively hard contouring (accompanied by the limited discriminability between the pontine tegmentum and the basis pontis) on quantitative postmortem fetal MR data, especially at very early stages of brain development. However, consideration was given to this region when comparing quantitative MR imaging and histology (Fig 5). Nevertheless, even though efforts were made to provide radiologic-pathologic correlations within the presented work, this study lacks a systematic comparison between histology-based myelin assessment and imaging-derived mapping. Foremost, the variability of the timeframe between the initiation of medically indicated termination of pregnancy (accompanied by the differences in the time spent in utero in each individual case) and state-of-the-art specimen work-up (eg, appropriate specimen fixation before onsets of postmortem lysis and, concomitantly, consistency-related issues, and so forth), prohibited high-quality myelin staining and, therefore, the acquisition of sufficient data for extended histologic (versus MR-based) analysis in most included cases. Besides these abovementioned limitations, advanced myelin staining (eg, immunohistochemical detection of myelin basic protein [MBP]) for the evaluation of early maturational stages was not routinely performed in postmortem fetal specimens. Finally, the number of included cases was relatively small. However, the investigated data represent a highly exclusive and rare sample.
CONCLUSIONS
Synthetic MR imaging and MRF provide highly sensitive imaging markers with which prenatal myelin development may be monitored on a quantitative, noninvasive basis. While the currently applied standard-of-reference MR contrasts and qualitative evaluation strategies of fetal imaging data do not allow a reliable assessment of ongoing myelinogenesis in utero, the strong correlations between myelin-related metrics and individual maturational stages observed in this study indicate the potential of quantitative MR imaging to identify delayed or abnormal states of white matter maturation. Therefore, quantitative imaging modalities represent promising tools with which to elevate antenatal diagnostics to a more objective level and to increase the capability to capture aberrances in myelin synthesis even at the earliest stages of cerebral development. These modalities represent further possibilities to improve state-of-the-art medicine and counseling of expectant mothers.
Footnotes
↵# V.U. Schmidbauer and I.-V.M Houech contributed equally and share first authorship.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received February 2, 2024.
- Accepted after revision April 23, 2024.
- © 2024 by American Journal of Neuroradiology