Graphical Abstract
SUMMARY:
With full FDA approval and Centers for Medicare & Medicaid Services coverage of lecanemab and donanemab, a growing number of practices are offering anti-amyloid immunotherapy to appropriate patients with cognitive impairment or mild dementia due to amyloid-positive Alzheimer disease. The goal of this article is to provide updated practical considerations for radiologists, including implementation of MR imaging protocols, workflows, and reporting and communication practices relevant to anti-amyloid immunotherapy and monitoring for amyloid-related imaging abnormalities (ARIA). On the basis of consensus discussion within an expanded American Society of Neuroradiology (ASNR) Alzheimer, ARIA, and Dementia Study Group, our purpose is the following: 1) summarize the FDA guidelines for the evaluation of radiographic ARIA; 2) review the 3 key MRI sequences for ARIA monitoring and standardized imaging protocols on the basis of ASNR-industry collaborations; 3) provide imaging recommendations for 3 key patient scenarios; 4) highlight the role of the radiologist in the care team for this population; 5) discuss implementation of MRI protocols to detect ARIA in diverse practice settings; and 6) present the results of the 2023 ASNR international neuroradiologist practice survey on dementia and ARIA imaging.
ABBREVIATIONS:
- AD
- Alzheimer disease
- APOE
- apolipoprotein-E
- ARIA
- amyloid-related imaging abnormalities
- ASNR
- American Society of Neuroradiology
- CMS
- Centers for Medicare & Medicaid Services
- GRE
- gradient-recalled echo
Monoclonal antibody therapies are becoming widespread in clinical practice, treating neurologic diseases ranging from cancer to MS and now Alzheimer disease (AD).1⇓-3 In AD, treatment with anti-amyloid immunotherapy may be associated with radiographic findings resembling cerebral amyloid angiopathy–related inflammation and has been termed amyloid-related imaging abnormality (ARIA).4⇓⇓⇓⇓⇓⇓⇓-12 ARIA is hypothesized to arise from the removal of amyloid deposits from and alongside cerebral arteries, resulting in vessel leakage and potentially provoking an immune response that can continue for weeks to months, even after cessation of anti-amyloid immunotherapy.13,14
The 2 types of ARIA are ARIA-E, in which -E stands for vasogenic edema and sulcal effusions, and ARIA-H, in which -H stands for hemorrhage and includes microhemorrhages and superficial siderosis that, in rare cases, may be associated with intraparenchymal hemorrhage and intraventricular hemorrhage. ARIA is a common adverse effect of anti-amyloid immunotherapy for AD, occurring in approximately 10%–30% of all participants treated in clinical trials of aducanumab, lecanemab, and donanemab.6,9,11,12 The data from those trials suggest that 74%–97% of ARIA is asymptomatic (ie, is detected on routine monitoring). It is rare for ARIA to present with neurologic symptoms, but when it does, symptoms can include headache, confusion, dizziness, visual changes, or praxis difficulties.8,9,11,15,16 Differentiation of symptomatic ARIA from stroke is a critical concern, because misdiagnosis and treatment with thrombolytics can potentially result in fatal intracranial hemorrhage.17
The primary known risk factor for ARIA is apolipoprotein-E (APOE) ε4 genotype carrier status, which is also associated with an increased risk of cerebral amyloid angiopathy, due to increased vascular amyloid deposits in APOE ε4 carriers.13 The effect of APOE ε4 on ARIA risk is dose-dependent, with APOE ε4 homozygous individuals displaying the highest risk. Baseline (pretreatment) microhemorrhages are also predictive of future ARIA-H, and patients with ≥5 baseline microhemorrhages were excluded from recent clinical trials using anti-amyloid immunotherapies.8,11,18 The second major risk factor for ARIA is drug dose. Finally, the risk of ARIA is associated with treatment duration. Patients are at greatest risk of developing ARIA during the first few months of treatment, when vessel wall amyloid is being mobilized.13 For these reasons, gradually increasing doses to reach a therapeutic dose level has been proposed to reduce ARIA incidence. Multiple noncontrast brain MRI scans are recommended to monitor ARIA, especially during treatment initiation.
The US FDA has adopted a standard radiographic classification for ARIA (Fig 1), following the classification systems used in clinical trials for anti-amyloid immunotherapies. The Alzheimer Disease and Related Disorders Therapeutic Workgroup (American Neurologic Association) has published a white paper recommending that anti-amyloid immunotherapy be paused for moderate ARIA-E or ARIA-H (even if asymptomatic) and discontinued for severe ARIA-H.19,20 Given these serious patient care implications, the potentially numerous MR imaging sessions needed, and the prevalence of patients with AD who may be treated, it is important for radiology practices to be prepared to perform, interpret, and communicate findings of these MRI examinations reliably, meaningfully, and in a timely manner.
ARIA radiographic severity score. ARIA-E, ARIA-H microhemorrhages, and ARIAI-H superficial siderosis are graded separately on the basis of treatment-emergent imaging findings. Any new, transient edema/sulcal effusion and new microhemorrhages or siderosis that occur while on treatment constitute ARIA. For ARIA-E, the size indicates the greatest extent of contiguous signal abnormality/gyral swelling measured in any dimension. Mild ARIA-E: new postdosing left parietal parenchymal edema measuring less than 5 cm (line); Moderate ARIA-E: new edema and gyral swelling in the right temporal-occipital lobes measuring 5–10 cm (line); and Severe ARIA-E: new edema and gyral swelling involving the left temporal-occipital lobes measuring greater 10 cm in greatest dimension. Mild ARIA-H microhemorrhages: one right occipital treatment-emergent microhemorrhage (arrow); Moderate ARIA-H: 6 scattered treatment-emergent microhemorrhages (arrows); and Severe ARIA-H: more than 10 treatment-emergent microhemorrhages clustered in the left occipital lobe (oval). Mild ARIA-H superficial siderosis: one right occipital region of treatment-emergent siderosis (oval); Moderate ARIA-H: 2 regions of siderosis (left sylvian fissure and left occipital, ovals); and Severe ARIA-H: 3 regions of siderosis (bilateral frontal and central sulcus, ovals). Some images courtesy of Dominantly Inherited Alzheimer’s Network (DIAN).
With ongoing studies and approval, AD therapeutics are becoming widely available in the community. As radiology practices prepare to provide care for these patients, many are seeking additional resources and guidance to optimize imaging protocols and workflows and to feasibly translate experience from clinical trials into clinical practice. This review article builds on our prior article, “Amyloid-Related Imaging Abnormalities with Emerging Alzheimer Disease Therapeutics: Detection and Reporting Recommendations for Clinical Practice.”21 The primary aim is to provide updated practical considerations for implementation of MR imaging protocols, workflows, and reporting and communication practices, on the basis of consensus discussion within an expanded American Society of Neuroradiology (ASNR) Alzheimer and Dementia Study Group, representing a broad scope of neuroradiology practice settings and locations.
Role of the Radiologist in the Patient Care Cycle
With the full FDA approval and Centers for Medicare & Medicaid Services (CMS) coverage of lecanemab and donanemab, anti-amyloid immunotherapy is now a realistic option for appropriate patients with mild cognitive impairment or early dementia due to amyloid-positive AD. Amyloid positivity can be determined by amyloid PET, as performed in the lecanemab Phase III trial;11 CSF biomarkers, as is most common in clinical practice;22 and plasma biomarkers, which are an emerging clinical option.23 The need to evaluate amyloid status, evaluate for exclusionary MRI findings at baseline, and perform safety monitoring has resulted in an increase in radiology services, including MRI scans and fluoroscopic-guided lumbar punctures. The recently updated CMS payment decision for amyloid PET, removing the requirement to be part of a clinical research study, will also likely increase the demand for amyloid PET imaging. The increased volume of MRI scans reflects a combination of baseline/enrollment scans to confirm eligibility for therapy based on several features, routine examinations to monitor for ARIA, examinations to follow-up documented ARIA, and imaging of symptomatic patients, with at least 4 MRI scans per patient anticipated in the first year of treatment.
The increase in scan volumes is expected to be widespread across academic and private practices, though it may vary on the basis of the local patient and provider population. Estimates of the number of patients who will undergo evaluation and treatment in a region and the projected increase in MRI scan volume may help facilities gauge their ability to accommodate the increased imaging demands for this patient population. Some practices have found that their projections may represent overestimates, given variable access to upstream elements in the care pathway (ie, referral to a neurologist) and other factors (eg, medical comorbidities, patient preferences). For example, in a recent study of participants with mild AD in a population-based sample, only 5%–8% of participants met the eligibility criteria for the aducanumab and lecanemab trials.24 Despite the limitations of projections, it is anticipated that with drug availability and patient interest, there could be large numbers of patients seeking to be evaluated for treatment. Each practice must decide how they will manage and triage these requests, which could easily overwhelm existing radiology capacities and workflows without careful planning. Close communication among referring providers, radiologists, and hospital administrations will aid in preparation. In this article, we provide recommendations for efficient and effective imaging of these patients, which can help accommodate the increased volumes.
Imaging Workflows: 3 Key Patient Scenarios
There are several aspects of radiology workflows that will benefit from advanced planning and consideration regarding AD therapeutics. We will discuss general considerations as well as those specific to 3 patient scenarios: 1) baseline dementia diagnosis/treatment enrollment evaluation, 2) asymptomatic ARIA monitoring, and 3) evaluation of the symptomatic patient on anti-amyloid immunotherapy. The applicability of these considerations to each practice will vary with the size of the practice, the ability to perform subspecialized examinations, and the diversity of the MRI scanners available.
Timing of MRI Scans
Each patient requires an MRI before the initiation of treatment to evaluate exclusionary findings and provide a baseline for future comparison. Appropriate use criteria by the Alzheimer Disease and Related Disorders Therapeutics Work Groups and the FDA label for aducanumab recommend that the baseline MRI should be performed within 1 year before enrollment.19,20,25,26 The lecanemab and donanemab FDA labels specify a “recent” brain MRI before enrollment. We recommend that the baseline brain MRI be ideally performed within 3–6 months before enrollment, but a brain MRI within 12 months may be adequate if negative for microhemorrhages, aligning with the lecanemab appropriate use recommendations.19 The presence of 1–4 baseline microhemorrhages on a brain MRI performed 7–12 months before initiating therapy should trigger a repeat examination, because these patients are of high risk for additional microhemorrhages. Interval progression of these findings (more microhemorrhages) may affect the patient’s treatment eligibility or, if not identified pretreatment, may later be counted as ARIA-H. A repeat baseline scan may also be indicated if the scan was not performed with a dedicated dementia protocol (described below), including blood-sensitive sequences of sufficient diagnostic quality to identify cerebral microhemorrhages or siderosis. Therefore, poor-quality brain MRIs, despite being within the 12 months before therapy initiation, will often necessitate repeat baseline imaging, increasing the burden on radiology departments.
During the first year of anti-amyloid immunotherapy, 3–4 routine noncontrast brain MRI scans for ARIA surveillance are recommended. Patients should undergo routine monitoring brain MRI at a minimum before the fifth, seventh, and 14th infusions on the basis of the lecanemab FDA label and before the second, third, fourth, and seventh infusions on the basis of the donanemab FDA label.19,26 The exact schedule of MRI examinations is likely to have some variability with different anti-amyloid immunotherapies.20,25 We recommend that the referring provider schedule these monitoring examinations at a single imaging center, if possible, to provide a consistent MRI examination for comparison. The referring provider may also consider prospectively scheduling all these examinations at the time of therapy initiation to reduce ambiguity for patients and caregivers and ensure that access to ongoing monitoring is established at the outset. Patients who develop ARIA, whether symptomatic or asymptomatic, will require additional monthly MRI scans until resolution of ARIA-E and stabilization of ARIA-H.
After the first 6 months (14th infusion for lecanemab), ARIA is infrequent (71% ARIA detected on the first 2 monitoring MRIs)11 and the frequency of MRI monitoring examinations is not clearly defined. The lecanemab appropriate use recommendations suggest an additional MRI before the 26th infusion (week 52).19 There are currently limited data on long-term therapy and monitoring, and recommendations may evolve as further experience is gained in clinical trials and clinical practice.
Patients who are on treatment and develop neurologic symptoms will require an additional brain MRI to evaluate for the presence of ARIA, typically on an urgent or emergent basis. Note that these patients may present to emergency departments with stroke-like symptoms and could mistakenly be treated with thrombolytics on the basis of the standard head CT performed using stroke imaging protocols, which has been associated with at least 1 fatality.17 Thus, the radiologist should have increased vigilance when discussing findings on head CTs for patients on anti-amyloid immunotherapies and provide recommendations for further evaluation with MRI. Emergent MRI should be considered in patients for whom the differential includes acute stroke versus ARIA and endovascular or, rarely, thrombolytic therapy is being considered.27 If MRI is not available, ARIA-E must be in the differential, with acute ischemia as the etiology for hypodensity identified on head CT and as the etiology of the patient’s symptoms. Outside the scenario of acute stroke management, urgent MRI should be considered to evaluate for ARIA in the symptomatic patient on anti-amyloid immunotherapy. As with other aspects of patient care, management of symptomatic patients on therapy requires a coordinated approach among radiology, neurology/treating providers, and emergency medicine.
3 Key MRI Sequences: ASNR ARIA Consensus Sequences
In all scenarios in which a patient on anti-amyloid immunotherapy is imaged, the 3 critical sequences are T2* gradient-recalled echo (GRE) (to evaluate for ARIA-H), 2D or 3D T2 FLAIR (to evaluate for ARIA-E), and DWI (to differentiate ARIA-E from acute ischemia). We will describe the rationale and details for each of these sequences and how they may be incorporated into specific imaging protocols.
1) ARIA-H Detection: T2* GRE versus SWI.
In clinical trials, ARIA-H detection has been performed using high-quality axial T2* GRE sequences, and the existing ARIA-H severity scoring systems are based on counts made using that sequence. Clinical trials used a GRE rather than a SWI sequence, because there is less variation in the imaging across scanners and vendors and SWI is not available on all scanners. Specific details on the performance of this T2* GRE are published in Cogswell et al,21 American Journal of Neuroradiology (AJNR) in 2022. The most important of these is the TE, which should be 15–20 ms at 3T and 25–35 ms at 1.5T. Our recommendation is unchanged: Standardized axial T2* GRE with appropriate TE should be performed in all scenarios for patients receiving anti-amyloid immunotherapy—baseline/enrollment, asymptomatic ARIA monitoring, and evaluation of a patient on anti-amyloid immunotherapy presenting with new neurologic symptoms.
Despite this recommendation, we acknowledge that other heme-sensitive sequences may be used for ARIA-H assessment and result in interpretation challenges. First, some practices are planning to run both T2* GRE and SWI in all cases to aid interpretation; obtaining both sequences may be helpful and feasible if the time for acquisition does not interfere with patient scheduling. When both sequences are obtained, we suggest that the ARIA-H findings represent the integrated interpretation of all available data (a single ARIA-H severity score for each microhemorrhage and siderosis based on information from both sequences). Because SWI is more sensitive than GRE for the detection of heme products,28 the decision to include SWI should be made in collaboration with referring physicians because this could result in a more conservative treatment approach.
Second, invariably patients may present to facilities without dedicated imaging protocols for ARIA detection and potentially be scanned under protocols that use a fast, lower-quality T2* GRE or SWI. As always, the radiologist should interpret the images in the context of the entire examination, using additional sequences and prior studies for comparison. The total number of microhemorrhages or the degree of siderosis should represent the radiologist’s best interpretation, using all available information; referring providers will be less interested in the technique by which microhemorrhage is detected and more interested in change or stability. If the interpretation is potentially biased by technical differences, it is important to clearly state this bias in the report. This statement is the standard practice when interpreting any patient examination and is common in our assessments of other patient populations such as those with MS or brain metastases. Statements such as “but not new in retrospect” or “likely unchanged, given differences in technique” may be helpful. Similar to typical interpretations for studies of questionable new metastases, suggestions for follow-up can be made in equivocal cases.
2) ARIA-E Detection: T2-Weighted FLAIR.
Clinical trials have previously used 2D T2 FLAIR for ARIA-E detection. However, 3D T2 FLAIR is now standard in clinical protocols at some institutions. Our recommendation for T2 FLAIR imaging is unchanged: We support the use of either 2D or 3D T2 FLAIR, whichever can be routinely performed with high quality in the practice. If 3D T2 FLAIR is implemented, multiplanar reformation in the axial plane is recommended to facilitate interpretations when comparing it with scans from other institutions that may use an axial 2D acquisition. Given that 3D FLAIR sequences are more immune to flow artifacts in the CSF that might be confused with ARIA-E sulcal effusion,29 they may be preferred, especially for problem-solving.
3) Ischemia Detection: DWI.
DWI is recommended to evaluate acute ischemia and differentiate it from ARIA-E. If a practice routinely performs DTI, providing and interpreting the DWI trace images and ADC map is required, as is standard clinical practice.
Imaging Protocols: 3 Patient Scenarios, 3 Key Sequences
For the baseline/enrollment examination, we recommend a noncontrast brain MRI, including the ARIA consensus T2 FLAIR, T2* GRE, and DWI as well as other standard sequences in the practice’s dementia protocol (Table). For asymptomatic ARIA monitoring, the protocol may be simplified to just the 3 ARIA consensus sequences, which could be performed in an abbreviated imaging slot and allow greater access for patients if the MRI scheduling is a bottleneck in the local care pathway. For symptomatic patients, in addition to ARIA, a full differential diagnosis such as infarct, tumor/metastases, and infection must be considered; thus, the MRI protocol would be tailored on the basis of the clinical presentation. Ideally, this protocol would also include the 3 ARIA consensus sequences.
| Baseline/Enrollment Evaluation | Asymptomatic Monitoring | Symptomatic Patient on Therapy | |
|---|---|---|---|
| Order | MRI brain dementia without IV contrast (indication: AD therapy enrollment) | MRI brain without IV contrast(indication: AD therapy monitoring) | MRI brain without (and with) IV contrast (indication: AD therapy, new symptoms) |
| Protocol | AD therapy enrollment | AD therapy monitoring | AD therapy monitoring |
| Minimum sequences | 2D or 3D T2 FLAIRGREa ± SWIDWI3D T1T2 FSE | 2D or 3D T2 FLAIRGREa ± SWIDWI | 2D or 3D T2 FLAIRGREa ± SWIDWI ± additional sequences |
| Reporting template | AD therapy enrollment | AD therapy monitoring | AD therapy monitoring |
| Key findings | MicrohemorrhagesSiderosisWhite matter hyperintensitiesInfarcts | ARIA-E (edema, effusion)ARIA-H (new microhemorrhages, siderosis) | ARIA-EARIA-HOther acute findings |
| Recommended communication | Standard reporting | Mild ARIA: notification requiredModerate or severe ARIA: closed-loop communication | |
↵a GRE must be performed with an appropriate T: 3T TE = 15–20 ms, 1.5T TE = 25–35 ms.
Summary of recommendations based on 3 patient scenarios
Practices may choose to include additional sequences according to their imaging capabilities and preferences and for problem-solving individual cases. For example, a T2 sequence may be added to help troubleshoot indeterminate findings on T2 FLAIR and GRE (eg, blood vessels) on a monitoring examination. Note, postcontrast imaging is not recommended at baseline or in monitoring of asymptomatic patients, and its use may result in unwarranted costs, patient risks, and extended examination durations. However, for symptomatic presentations, contrast may be indicated for fully evaluating the differential diagnosis on the basis of the clinical presentations.
MRI Brain Orders
Because different protocols are recommended for each of the 3 patient scenarios described, obtaining the appropriate protocol may be facilitated by unique orders for each. Specific orders for the baseline/enrollment evaluation, asymptomatic ARIA monitoring, and symptomatic patients receiving anti-amyloid immunotherapy may be considered and linked to distinct imaging protocols and reporting templates.
For AD therapeutics baseline/enrollment evaluation, the order may trigger the examination code MRI brain dementia without IV contrast with the examination indication “AD therapeutics enrollment.” Using the same imaging protocols for the indication AD therapeutics enrollment and for the standard “dementia evaluation” may reduce the need for repeat imaging at the time of the baseline evaluation, before the initiation of anti-amyloid immunotherapy.
An “AD therapeutics/ARIA monitoring” order may trigger an examination code MRI brain without IV contrast with the indication “AD therapeutics monitoring.” For monitoring, an order specific to AD therapeutics could serve to indicate that the dedicated imaging protocol should be performed and, via examination indication, also alert the radiologist to the purpose of the examination. Ideally, the date of treatment initiation should be included in the clinical indication for each monitoring brain MRI to facilitate appropriate comparison with the baseline and most recent prior examinations.
For symptomatic patients on anti-amyloid immunotherapy, the AD therapeutics/ARIA monitoring order may be used, with additional sequences based on the clinical presentation. The examination indication will be particularly important when patients on treatment are being evaluated for new symptoms, for which the context of anti-amyloid immunotherapy is critical to distinguishing probable ARIA from radiologic mimics.
Field Strength
FDA labels do not specify a field strength for brain MRI examinations to monitor for ARIA. Clinical trial imaging has been largely performed at 3T, with imaging at 1.5T for a subset of sites and patients, 1.5T being the lowest field strength used in trials. Use of a lower field strength may result in underdiagnosis of ARIA-H because the effects of the magnetic susceptibility are proportional with field strength.28 Practitioners should be aware that microhemorrhages may only variably be detected on lower-field-strength scanners (eg, ∼0.5T or open scanners) and are not visible on current ultra-low-field scanners (eg, 0.06T). In summary, our recommendation for scanner field strength in AD therapeutics imaging is unchanged: 3T is recommended, 1.5T and greater is adequate, and less than 1.5T is inadequate. To increase the overall capacity and meet growing demand for MRI, further research regarding the detection of ARIA with lower field strengths would be helpful.
Standardization
Standardized imaging is important in ARIA evaluations because it facilitates the use of standardized assessment and treatment criteria and the ability to compare findings among serial MRI examinations in an individual patient. Ideally, a patient would be imaged with the same field strength, vendor, and scanner model, using the same sequences and sequence parameters across serial examinations (Fig 2). However, that level of consistency is typically not feasible in clinical practice, given that a patient may not be imaged at the same imaging center across serial visits, and even at a single imaging center, there may be a diverse assortment of MRI scanners. Imaging using the same MR vendor is important to prevent ARIA-E mimics due to the differential appearance of white matter hyperintensities among vendors.21 Imaging at the same field strength is more important for ARIA-H detection, given that the sensitivity for detecting heme products is proportional with magnetic field strength. The use of harmonized protocols (similar sequences and sequence parameters) across the scanners in a practice would standardize evaluation regardless of the scanner used.
Pyramid of MRI protocol standardization. Ideally, patients are imaged using the same sequences and sequence parameters across serial examinations, using the same field strength, vendor, and scanner model. If a patient cannot be imaged on the same scanner across serial examinations, radiologists must be aware of differences that may affect the ARIA evaluation. Most important, at the pyramid base, a standardized set of MRI sequences should be used. Next in importance is the field strength, because sensitivity for heme products varies proportionally. Third, a patient would ideally be imaged using the same scanner vendor to prevent ARIA mimics on the basis of the differential appearance of white matter hyperintensities among vendors.
To facilitate standardization, the ASNR has collaborated with GE HealthCare, Philips Healthcare, and Siemens to create standardized T2 FLAIR, T2* GRE, and DWI ARIA consensus protocols that can be directly imported into scanners through established vendor support. Each vendor has developed protocols based on the above recommendations and those provided in the prior publication by Cogswell et al21 (eg, 2D slice thickness of 4 mm) and harmonized the sequences across their collection of scanners. The base protocol from each vendor includes a 2D T2 FLAIR, T2* GRE, and DWI, which can be performed in <15 minutes of gradient time. The following are provided as optional sequences: 3D T2 FLAIR (may be used in place of 2D T2 FLAIR), SWI (may be used in addition to T2* GRE), and 3D T1. These protocols are posted on the ASNR Web site (https://www.asnr.org/education-resources/alzheimers-webinar-series/) and will be available in the product protocol trees. This harmonization is a major accomplishment for standardization in patient care and should help to decrease variability of interpretation of ARIA.
Interpretation and Reporting Workflows
Interpretation
Because ARIA is a new entity in the field of clinical imaging, education and training are important to ensure accuracy and consistency in reporting. Ongoing training and continuing medical education are available through the ASNR (https://www.asnr.org/education-resources/alzheimers-webinar-series/), the American College of Radiology, the Alzheimer’s Association, the Radiological Society of North America, pharmaceutical companies, and during specialty meetings.
Comparison with prior examinations (baseline and most recent prior) is necessary for ARIA assessment. Similar to other aspects of the evaluation, the comparison with prior examinations will be aided by the patient consistently receiving care in the same imaging system. If such consistency of care is not possible, prior imaging could be made available via a central patient registry, such as the Alzheimer’s Network for Treatment and Diagnostics (ALZ-NET) (alz-net.org). Additionally, patients may be encouraged to obtain a copy of their baseline (± subsequent) MRI examinations and have these studies available at monitoring visits. If the radiologist does not have a prior scan for comparison, a scan with negative findings is conclusive (no ARIA). However, accurate detection of mild ARIA-E will be hindered, and if microhemorrhages are present, an accurate ARIA-H microhemorrhage severity score would not be possible without comparison with the baseline MRI.
Reporting
We recommend the use of a reporting template (see Resources section at https://www.asnr.org/education-resources/alzheimers-webinar-series/), which will guide the reader through reporting of the relevant findings for baseline/enrollment and monitoring MRI examinations. An example of the temporal evolution of ARIA and sample ARIA reporting are shown in Fig 3 and the Online Supplemental Data.
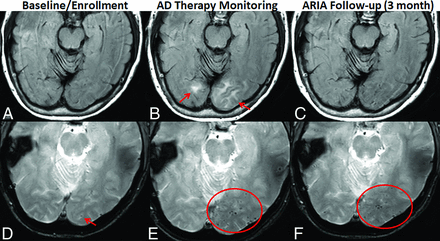
Temporal evolution of ARIA and reporting. AD therapy enrollment (A and D). Axial T2 FLAIR shows mild white matter hyperintensities (incompletely imaged in slice shown) and no infarcts (A). GRE shows one left occipital microhemorrhage (arrow) and no superficial siderosis (D). AD therapy monitoring (B and E). T2 FLAIR shows new T2 hyperintense signal and edema in the left-greater-than-right occipital white matter, measuring up to 3.6 cm in the greatest linear dimension. On the basis of 2 regions of signal abnormality, this finding is moderate ARIA-E (arrows, B). GRE shows a total of 13 new microhemorrhages (only some shown on this slice), severe ARIA-H microhemorrhages, and no ARIA-H siderosis (oval, E). The patient was followed with monthly MRIs until the resolution of ARIA-E and stabilization of ARIA-H, which occurred after 3 months (C and F). T2 FLAIR shows resolution of occipital T2 signal abnormality and no new FLAIR signal abnormality. No ARIA-E (C). GRE shows no new microhemorrhages. There is a total of 14 microhemorrhages, one unchanged from baseline, and 13 treatment-emergent. Unchanged severe ARIA-H (oval, F). See the Online Supplemental Data for sample reports of these findings. Images courtesy of Dominantly Inherited Alzheimer’s Network (DIAN).
For the baseline and enrollment MRI examination, the report should include the relevant exclusionary findings. The American Neurologic Association white paper recommends consideration of the number of baseline microhemorrhages, regions of superficial siderosis, number of lacunar infarcts and infarcts involving a major vascular territory, and the extent of baseline white matter hyperintensity, graded using the Fazekas scale.19,20,30 These can be combined in a single section of the radiology findings, as in the reporting templates.
For the ARIA monitoring examinations, the report must include all relevant findings to arrive at an ARIA severity score: treatment-emergent edema/sulcal effusion, cumulative numbers of treatment-emergent microhemorrhages and regions of superficial siderosis (Fig 1). These findings will be used along with clinical symptoms to determine the patient’s eligibility for continued drug dosing. In addition to the use of a reporting template, marking findings on examinations is helpful. Findings may be marked with an arrow or similar notation, labeled as “new” on the date first identified and/or listed with image numbers in the report to assist tracking across serial examinations. Clearly describing the number of pre-existing and new ARIA findings will help provide a total count; both may influence treatment decisions. If the radiologist cannot adequately assess ARIA, for example, due to patient motion or lack of or poor-quality GRE/SWI, that information should be stated in the report. Inclusion of standardized MRI sequences and reporting in the baseline and ARIA monitoring MRI examinations will provide the details needed for treatment decisions and can help to reduce the burden on the clinical practice by decreasing the number of “second reads” and repeat MRI examinations.
An example of a streamlined workflow integrating MRI orders, protocols, and templates is as follows: A unique orderable is created for both “AD therapy enrollment” (MRI brain dementia without IV contrast) and “AD therapy monitoring” (MRI brain without IV contrast). These orders specify a set of scanners on which examinations should be scheduled and indicate the appropriate imaging protocol. Finally, the use of a unique orderable allows the appropriate reporting template (AD therapy enrollment or AD therapy monitoring) to auto-populate when the examination is opened for interpretation.
Communication
The radiologist will play an important role in the decisions to enroll and maintain patients on treatment on the basis of relevant MRI findings. Communication between ordering providers and radiologists will be critical in all encounters, including baseline assessment, asymptomatic monitoring, and during work-up of symptoms, when the radiologist must entertain a broad differential diagnosis.
Nonroutine communication is recommended for findings of ARIA. When there is mild ARIA, notification is required (eg, automated message or communication via an assistant). Moderate-to-severe ARIA requires closed-loop urgent communication. This communication is important because ARIA findings along with clinical symptoms will be used to make treatment decisions, and there may be a mismatch between radiographic severity and symptoms. Either can trigger further necessary action.
Computer-Aided Detection
Artificial intelligence computer-automated detection solutions are in active development to aid neuroradiologists in ARIA reporting through the development of quantitative ARIA monitoring reports. The automated quantitative software may provide the location and count of new microhemorrhages and superficial siderosis (ARIA-H), as well as measure the greatest dimension and volume of edema/sulcal effusions (ARIA-E). The reports may provide longitudinal data across serial MRI scans and display criteria for grading ARIA as mild, moderate, or severe in the correct clinical construct. The ability to directly insert these findings into the radiologist’s dictation through an automated “prepopulated reporting template” may facilitate reporting. As always, these tools are considered an adjunct to neuroradiologic interpretation.
Practical Considerations
Patient access to MRI at timely intervals for follow-up imaging during treatment may be challenging at many sites if volumes increase as projected. Many practices are already facing limited MRI scanner slot availability due to pre-existing system overload secondary to a combination of increasing volume from multiple patient populations, a shortage of qualified MRI technologists to staff examinations, and aging MRI systems. To increase capacity, patients may receive ongoing infusions and MRI monitoring at a center different from the one for their initial evaluation on the basis of availability. It is important that radiologists adopt standardized acquisition and reporting protocols for MRI in this situation to avoid miscommunication between providers and provide optimal care for patients undergoing anti-amyloid immunotherapy, regardless of the practice setting.
One approach to improving access to MRI is to reduce the imaging time per patient, which may be achieved by limiting the number of sequences acquired or reducing the time per sequence. As discussed above, conventional brain imaging protocols may be truncated to include only the most essential sequences for ARIA assessment (T2 FLAIR, T2* GRE, and DWI) (Table). To reduce sequence time, practices may consider implementing accelerated MR imaging techniques, such as compressed sensing and/or artificial intelligence–based reconstructions.31,32
Another challenge practices will face will be how to handle patients with special MRI requirements, such as claustrophobia, cardiac implantable electronic devices, spinal stimulators, and other implanted devices. These examinations typically necessitate specialized imaging personnel such as an MR safety officer or MR physicist safety expert to perform them safely and are not easily applied to large populations. Therefore, per appropriate use recommendations,27 patients unable to undergo a routine brain MRI may be excluded from treatment. However, in practices with the required resources, imaging and treatment of such patients may be feasible, likely at 1.5T, provided the ability to obtain emergent imaging in case of potential adverse effects is clarified. In these cases, coordinated care among the referring provider, patient, and radiologist will be needed to assess the risk and benefit.
Integrated Care
Coordinated care among multidisciplinary providers will be important to provide the best patient care and streamline evaluation. In this article, we focused on the brain MRI, one of multiple tests performed to evaluate patient eligibility for anti-amyloid immunotherapy. In the setting of a coordinated evaluation, MRI would best be performed early in the evaluation before amyloid status assessment so that if there are exclusionary findings on MRI, the more costly and/or invasive amyloid PET or lumbar puncture may be avoided. Once the data are in hand, providers must determine patient eligibility for treatment, which will include integration of AD biomarker data, brain MRI findings, and a clinical examination, among others. Multidisciplinary input may be particularly valuable as providers begin to navigate the application of the inclusion/exclusion criteria and appropriate use recommendations in real-world clinical practice. The degree to which such integration of care can be achieved will necessarily vary across different centers, depending on availability of clinical and imaging resources.
ASNR ARIA Survey Results
In July 2023, a survey was sent to all ASNR members with the goal of gauging the radiology community’s preparedness and capacity to image patients receiving anti-amyloid immunotherapies. There was a total of 221 respondents, of whom, 154 (69.7%) practiced in academic medical centers. Respondents reported variable degrees of confidence in the ability to identify ARIA on a brain MRI (Fig 4). Less than one-half of respondents (39.4%) had specific MRI protocols in place for AD therapeutics imaging. For the practices with protocols in place, most planned to use 3T (90.2%) and 1.5T (70.7%) field strength scanners, and imaging sequences included in the protocol varied among practices. See the Online Supplemental Data and figures for the full survey results.
Selected results from the 2023 ASNR survey gauging practice readiness for AD therapeutics imaging. A, How confident are you in your ability to identify ARIA on a brain MRI? B, Do you (your practice) have specific imaging protocols in place for AD therapeutics imaging? C, At what field strengths will your practice perform AD therapeutics imaging? D, What sequences are included in your AD therapeutics imaging protocol?
CONCLUSIONS
The advent of disease-modifying treatments for AD heralds a new era of hope for patients with early dementia due to AD and those with mild cognitive impairment and their families. Treatment with anti-amyloid immunotherapy requires multiple brain MRIs. To assist radiology practices with these new demands, we have outlined 3 patient scenarios: baseline/enrollment, asymptomatic ARIA monitoring, and the symptomatic patient on therapy. In each of these 3 scenarios, it is important to obtain 3 key brain MRI sequences (T2 FLAIR, T2* GRE, and DWI) that can be implemented in a standardized fashion to improve access to care and to standardize interpretation of results. In partnership with industry, these are now available from each of the major MRI vendors. We have also updated the prior ASNR recommendations to specifically address questions regarding workflows, reporting, communication of findings, specific patient scenarios that may require individualized care, and future directions for research. These efforts address the most pressing needs of the neuroradiology community, as indicated by our recent survey. As we continue to gain experience and knowledge with these therapies, we will share that information and update these imaging recommendations with the community via future publications and webinars.
Acknowledgments
We thank our colleagues at GE HealthCare, Philips Healthcare, and Siemens for their collaboration in providing a standardized protocol for AD therapeutics imaging and Biogen and the Dominantly Inherited Alzheimer’s Network (DIAN) for images.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received April 17, 2024.
- Accepted after revision August 12, 2024.
- © 2025 by American Journal of Neuroradiology