Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: The quality of resting-state fMRI (rs-fMRI) under anesthesia is variable and there are no guidelines on optimal image acquisition or anesthesia protocol. We aim to identify the factors that may lead to compromised clinical rs-fMRI under anesthesia.
MATERIALS AND METHODS: In this cross-sectional study, we analyzed clinical rs-fMRI data acquired under anesthesia from 2009–2023 at Massachusetts General Hospital. Independent component analysis–driven resting-state networks (RSNs) of each patient were evaluated qualitatively and quantitatively and grouped as robust or weak. Overall networks were evaluated by using the qualitative method, and motor and language networks were evaluated by using the quantitative method. RSN robustness was analyzed in 4 outcome categories: overall, combined motor-language, individual motor, and language networks. Predictor variables included rs-fMRI acquisition parameters, anesthesia medications, underlying brain structural abnormalities, age, and sex. Logistic regression was used to examine the effect of the study variables on RSN robustness.
RESULTS: Sixty-nine patients were identified. With qualitative assessment, 40 had robust and 29 had weak overall RSN. Quantitatively, 45 patients had robust, while 24 had weak motor-language networks. Among all the predictor variables, only sevoflurane significantly contributed to the outcomes, with sevoflurane administration reducing the odds of having robust RSN in overall (OR = 0.2, 95% CI = 0.05–0.79, P = .02), motor-language (OR = 0.18, 95% CI = 0.04–0.80, P = .02), and individual motor (OR = 0.1, 95% CI = 0.02–0.64, P = .02) categories. Individual language network robustness was not associated with the tested predictor variables.
CONCLUSIONS: Sevoflurane anesthesia may compromise the visibility of fMRI RSN, particularly impacting motor networks. This finding suggests that the type of anesthesia is a critical factor in rs-fMRI quality. We did not observe the association of the MR acquisition technique or underlying structural abnormality with the RSN robustness.
ABBREVIATIONS:
- BOLD
- blood oxygen level–dependent
- rs-fMRI
- resting-state fMRI
- RSN
- resting-state network
SUMMARY
PREVIOUS LITERATURE:
Limited literature is available on the effect of accelerated resting-state fMRI (rs-fMRI) and other imaging parameters on the quality of rs-fMRI in clinical setting where visibility of surgically relevant networks is crucial. Most of the prior work has used nonaccelerated techniques. Vast literature on rs-fMRI under anesthesia has focused on functional connectivity metrics to arrive at neuronal correlates of anesthesia, rather than clinical metrics that can be used for individual patients. The literature on the optimal anesthesia protocol for rs-fMRI is mixed with conflicting findings.
KEY FINDINGS:
Sixty-nine clinical rs-fMRI examinations under anesthesia were evaluated retrospectively for network activation robustness. Among all the tested variables, including accelerated and nonaccelerated MR techniques, scan length, structural abnormalities, and multiple anesthesia medications, only sevoflurane was associated with weak networks.
KNOWLEDGE ADVANCEMENT:
Sevoflurane may compromise the visibility of MRI resting-state networks under anesthesia, mainly the motor network, without affecting the language network.
Noninvasive functional brain mapping in young children and cognitively impaired patients is challenging with standard awake task-based fMRI, which requires patient cooperation, and effort. Resting-state fMRI (rs-fMRI) is task-free and can address this challenge by revealing multiple functional brain networks without a need for direct patient participation.1,2 However, rs-fMRI under anesthesia, which is often required for this patient population, is technically challenging and is prone to failure.3 The use of rs-fMRI is expanding in clinical settings, currently complementing task-based fMRI and invasive methods such as direct electrical cortical stimulation. The development of new reliable analytical methods, combined with the unique insights rs-fMRI provides about functional anatomy, makes this technique promising as a stand-alone clinical tool in the future, particularly for patients who cannot undergo traditional methods.4⇓-6 Optimizing rs-fMRI is essential for noninvasive personalized treatment planning in neurologic disorders in this patient population. In this study we aim to identify the factors that may lead to compromised clinical rs-fMRI under anesthesia.
Blood oxygenation level–dependent (BOLD) imaging is the main technique used for rs-fMRI.7 Synchronous fluctuation of the BOLD signal in distant parts of the brain at rest form functionally connected resting-state networks (RSNs).2 BOLD signal is an indirect marker of neuronal activity and depends on a phenomenon called neurovascular coupling where neuronal activity leads to increased blood flow.8 Decreased intrinsic neuronal activity or neurovascular uncoupling may degrade fMRI ability to reveal RSN. Cerebral blood flow is decreased under anesthesia as is the intrinsic signaling of certain parts of the brain depending on the anesthetic,9,10 which may lead to neurovascular uncoupling or drop of BOLD signal below detection levels.
Functional MR sequences under anesthesia need to be tailored to capture this suppressed signal. Accelerated rs-fMRI is a desired technique for its fast acquisition, high temporal resolution, and low motion artifact. The trade-off is a low SNR in each BOLD volume compared with nonaccelerated fMRI. In awake imaging, moderately accelerated rs-fMRI can detect resting BOLD signal fluctuation.11 However, under anesthesia, a low SNR from acceleration may outweigh the benefits. In sedated animal studies accelerated rs-fMRI has been used,12 but in humans, the key studies did not use accelerated rs-fMRI.13⇓⇓⇓-17 We hypothesize that acceleration may compromise rs-fMRI under anesthesia in humans.
The literature on neurovascular coupling in sedated humans is limited18 and the optimal anesthetic regimen to produce visible networks in rs-fMRI is unknown. Animal studies favor a combination of medetomidine and isoflurane anesthetic agents for rs-fMRI.19 In humans, the commonly studied anesthetics (sevoflurane and propofol) decrease connectivity of thalamocortical and frontoparietal networks.10,20,21 Nonetheless successful utilization of these agents has been reported in pilot clinical studies.13,14,20⇓⇓⇓⇓-25 Given our institutional experience, we hypothesized that propofol compromises the RSN further than other agents.
To test our hypotheses, we evaluated the impact of different MR acquisition parameters and anesthesia medications on the quality of clinical rs-fMRI under anesthesia in individual patients.
MATERIALS AND METHODS
Study Design
We conducted this retrospective, cross-sectional study by using human subject data with institutional review board approval (#2021A019008). Informed consent was waived with institutional review board approval.
Setting.
The research was carried out in a tertiary adult and pediatric care hospital, utilizing the hospital’s extensive database of rs-fMRI, acquired as part of institutional battery of functional MR sequences.
Participants
Consecutive patients of all age groups and all pathologies who underwent rs-fMRI under anesthesia at Massachusetts General Hospital from 2009–2023 were included in the study. The patients were excluded if the sedation was not done for image acquisition purposes, as in sedated or comatose intensive care unit patients. Patients who did not have a structural MRI performed during the same session as the rs-fMRI were excluded. Patients with hematoma that would interfere with the image analysis were excluded. The functional imaging was requested by the referring provider in each of the subjects.
Variables.
Outcome variables are rs-fMRI network quality (robust versus weak) as defined in the following sections. Predictor variables, also detailed in the following sections, include fMRI acquisition parameters (multiband versus regular, scan length, and number of BOLD volumes), anesthesia medications (propofol, sevoflurane, N2O, and other), underlying structural abnormalities, age, and sex.
Data Sources/Measurement.
Anesthesia.
The decision to administer anesthesia during MRI acquisition was made clinically and not influenced by this study. The type of anesthesia was chosen by the anesthesiologist and individualized for each patient. One or more medications were used for each patient without a particular pattern. Frequently used medications for our study group (sevoflurane, propofol, and N2O) were each considered as a predictor variable. Less frequently used medications (eg, fentanyl and dexmedetomidine) constituted the “other medication” variable. The primary investigator retrieved the data directly from the anesthesia event documented at electronic medical record by the anesthesiologist.
MRI Acquisition.
All scans were performed on a Siemens (Prisma fit or TrioTim) 3T MRI scanner. Anatomic images were obtained by using MPRAGE 3D T1-weighted sequences (TE: 2.3 ms, TR: 2300 ms, flip angle: 8, voxel size: 1 × 1 × 1 mm3). Resting-state BOLD fMRI was obtained by using a 2D echo-planar imaging either with acceleration (TE: 36 ms, TR: 1250 ms, flip angle: 60, axial pixel size: 2 × 2 mm2, slice thickness 2 mm, multiband factor: 5), or without acceleration (TE: 30 ms, TR: 3000 ms, flip angle: 90, axial pixel size: 2 × 2 mm2, slice thickness 3 mm). All the scans from the TrioTim scanner were without acceleration. The rs-fMRI scan lengths ranged between 5–10 minutes in 1 or multiple runs, and total BOLD volumes ranged between 90–550 per subject. Given all our subjects were under anesthesia, there was no control on eyes open or eyes closed during rs-fMRI acquisition. rs-fMRI sequences were acquired before contrast administration. No passive task-based fMRI was performed during the scans.
We documented the presence of acceleration technique for fMRI (multiband versus regular), scan length, and number of BOLD volumes as the 3 MR-related predictor variables.
Image Analysis.
Preprocessing was performed by using fMRIPrep26 at participant level. Initially, raw data underwent motion correction by using a rigid body transformation to realign the time-series. Brain extraction (skull-stripping) was performed by using the MNI152NLin2009cAsym template. Slice timing correction was not performed. Data were then spatially normalized to the MNI152NLin2009cAsym template at 2-mm resolution, the native T1-weighted anatomic space, the native FreeSurfer anatomic space, and the FreeSurfer average surface space (fsaverage6). Noise reduction was applied by using CompCor to mitigate physiologic noise, and confound regression included motion parameters, global signals, and Anatomic CompCor components. Spatial smoothing was not applied at this stage and was performed during independent component analysis. ICA by using FSL MELODIC (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC) software27 was used for functional connectivity analysis and network delineation. Mixture modeling on Z-transformed independent component maps was performed for multiple comparison correction. Assessment of RSN robustness was performed by using qualitative and quantitative methods.
Qualitatively, a neuroradiologist (E.H., with 2–3 years of subspecialty experience) grouped each patient’s surgically relevant RSN into overall robust or overall weak. At the time of grouping, the neuroradiologist (E.H.) was blind to the study variables including anesthesia medication. Similar to clinical practice, patient-specific visual thresholding was used to reveal meaningful RSN activation maps. Language, sensorimotor, visual, and auditory RSN were considered surgically relevant, while default mode, salient, attention, and executive function networks were considered not relevant. An overall robust RSN was defined as large cortical activations within boundaries of 2 or more surgically relevant networks. An overall weak RSN was defined as absent, small or <2 surgically relevant network activations.
For the quantitative analysis, the preprocessed data underwent further analysis with an in-house Matlab (MathWorks) script built on established techniques detailed previously with modification.4 Briefly, a template matching process with Discriminability Index-based Component Identification score analyzed the ICA components and created a motor and a language map for each patient in their native brain space. A visual quality assurance step was performed by a neuropsychologist (A.A., with 3–5 years of functional brain mapping and analysis) to ensure the selected maps were accurate. In cases of algorithm failure, for example if a noise component was selected as the functional map, the algorithm was adjusted to select the next best map. Next, for each of the language and motor maps, we computed a separate Dice coefficient to quantify the extent of overlap between the map and the corresponding template. The combined motor-language network quality for each patient was considered “robust” if Dice coefficients for both motor and language maps were above 0.5, otherwise “weak.” Similarly for individual analysis of motor and language networks, robustness was defined as Dice coefficient above 0.5. The decision to use Dice coefficient cutoff of 0.5 was based on our review of the data in this sample and general guidelines that consider the coefficient of 0.5 as fair overlap. On retrospective evaluation, this cutoff appropriately identified the cases in our study population with no meaningful RSN.
The following rs-fMRI network robustness outcome variables were documented: overall (qualitative), motor-language (quantitative), motor (quantitative), and language (quantitative).
Motion Parameters.
The mean framewise displacement at the individual patient level was recorded from fMRIPrep derivatives and used for analysis. Framewise displacement was calculated as the sum of the absolute values of the differentiated realignment estimates, including both translational (x, y, z) and rotational (pitch, roll, yaw) movements.
Bias.
Analysis bias was addressed by the addition of quantitative steps. Qualitative analysis bias was decreased by blinding the interpreting neuroradiologist to the study variables at the time of grouping the RSN.
Statistical Analysis.
Logistic regression was used to determine the association of study variables (MRI parameters, anesthesia medication, age, sex, and presence of structural abnormality) with robustness of RSN in 4 defined categories (overall, motor-language, motor, and language). Variance inflation factor was used to quantify the level of multicollinearity among the variables. We intended to keep all the predictor variables in the final regression model. However, we removed BOLD volume variable given its collinearity with accelerated MRI variable. In addition, we performed polynomial regression for continuous variables where we did not observe the effect of higher orders, thus confirming the linear assumption for our model, precluding the need for sensitivity analysis. ORs were calculated to measure the strength of these associations, with P values < .05 considered statistically significant. Missing data were excluded from the analysis and are reported as count and percentage. Comparison of mean motion parameter between groups was done by using independent sample t test. R software (R Version 4.0.1, http://www.r-project.org) and SPSS (Version 28.0, IBM) was used for analysis (M.H. and Z.L.). The sample size was determined based on the availability of eligible patients within the study period. Post hoc power analysis was done by using type I error rate (α) = .05, and sample size of 69. The null hypothesis was set to sevoflurane OR = 1 (no effect) in each outcome category. Alternative hypotheses were set to the observed sevoflurane ORs in our data in each outcome category (ie, overall RSN OR = 0.2, motor-language OR = 0.18, and individual motor OR = 0.1, as seen in the following Results section). The analysis was performed on G*Power software (Version 3.1.9.7).
RESULTS
Participants and Descriptive Data
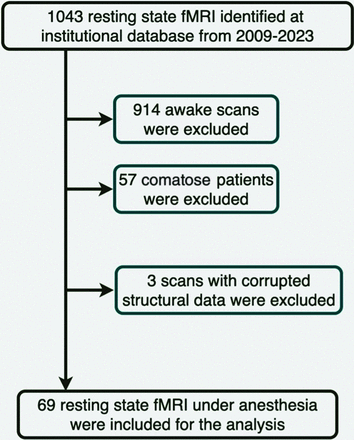
A total of 69 scans from 69 patients (mean age = 6.9 ± 4.3, 39 boys) were included in the study (Fig 1). Detailed demographic information, imaging protocols and anesthesia types are shown in Table 1. Most (59%) of the rs-fMRIs were performed without acceleration. Anesthesia medications included propofol (84%), sevoflurane, N2O, and other (fentanyl, dexmedetomidine). Approximately 43% of the structural examinations were normal. The structural abnormalities seen on the remainder included, but were not limited to, nonspecific white matter lesions, tumor, encephalomalacia, hemorrhage, abnormalities of gyration and sulcation (eg, polymicrogyria), and mesial temporal sclerosis. Indication for the examination was primarily epilepsy/seizure (51% of the cases) but also included traumatic brain injury, developmental delay, stroke, and headache.
Flow diagram of patient selection.
Demographics, clinical, and imaging characteristics of study population
Outcome Data
With qualitative assessment, 40 patients (58%) had overall robust RSN and 29 (42%) had weak networks. With quantitative assessment, 45 patients (65%) had robust combined motor-language networks while 24 (35%) had weak networks. With individual assessment of motor and language, 57 patients (83%) had robust language network and 52 patients (75%) had robust motor network. Examples of robust and weak RSN in our study population are shown in Figs 2 and 3.
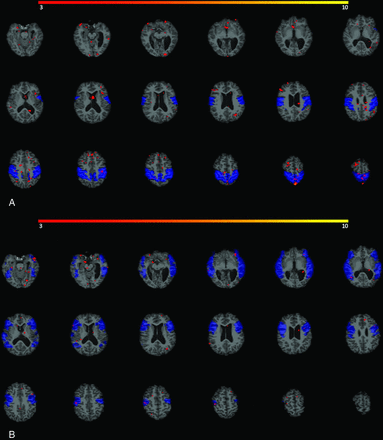
Examples of robust RSNs in our study population. Network activations are depicted in red-yellow spectrum. A, Robust motor network activation is seen (both visually and quantitatively) in an 8-year-old boy with epilepsy who underwent a 10.4-minute rs-fMRI with acceleration under propofol anesthesia. Structural images showed chronic right MCA infarction. B, Robust visual network activation is seen in a 9-year-old boy with epilepsy who underwent a 7.25-minute rs-fMRI without acceleration under propofol anesthesia. No structural abnormality was detected. C, Robust language network activation is seen (both visually and quantitatively) in a 5-year-old girl with seizure who underwent a 6.75-minute rs-fMRI without acceleration under propofol, sevoflurane, and fentanyl anesthesia. Subacute infarction in the right MCA territory was seen on structural images.
Example of weak RSNs in a 9-year-old boy with seizure who underwent 7-minute rs-fMRI under propofol, sevoflurane, and N2O anesthesia. Structural images showed mesial temporal sclerosis, and chronic left germinal matrix hemorrhage. No meaningful activation (depicted in red) is seen in motor network (A) or language network (B) boundaries, shown as blue template masks.
Main Results
Among all the tested variables, only sevoflurane was significantly associated with the quality of RSN; with sevoflurane use reducing the odds of having robust overall RSN (OR = 0.2, P = .02), combined motor-language networks (OR = 0.18, P = .02), and individual motor network (OR = 0.1, P = .02) as detailed in Tables 2, 3, and 4. None of the tested variables were associated with weak individual language network (Table 5).
ORs and CIs from multivariable logistic regression analysis of predictive factors in robust rs-fMRI overall networks with qualitative assessment
ORs and CIs from multivariable logistic regression analysis of predictive factors in robust rs-fMRI motor-language networks with quantitative assessment
ORs and CIs from multivariable logistic regression analysis of predictive factors in robust rs-fMRI motor network with quantitative assessment
ORs and CIs from multivariable logistic regression analysis of predictive factors in robust rs-fMRI language network with quantitative assessment
Other Analysis
Negligible motion was observed in all of the 69 examinations (Table 1). No significant difference was noted between the mean framewise displacement parameter between the patients who received sevoflurane (mean = 0.05 ± 0.03) compared with the group who did not (mean = 0.06 ± 0.04) (P = .35). In a post hoc power analysis, our sample size achieved a power of 80% and above to detect statistically significant results.
DISCUSSION
This study evaluated the factors that might have a role in the quality of clinical rs-fMRI under anesthesia and found that sevoflurane anesthetic is associated with weak overall RSNs and motor network individually. Our result did not show association of other factors, including image acquisition parameters (multiband acceleration or scan length) or other anesthetics (propofol or N2O) with network robustness. Of note, language network robustness was not associated with any of the tested variables.
We used clinically relevant outcome measures and individual patient-level analysis, enhancing clinical translation compared with previous literature that mainly used functional connectivity metrics.14,20 These metrics, while essential in advancing knowledge about neuronal correlate of anesthesia, are less useful for individual patients. We bridged this gap with various measures. First, akin to task-based fMRI, we applied visual inspection of statistical maps, integrating knowledge about eloquent cortical regions associated with the evaluated functions. Second, we focused on motor and language networks, which are paramount in presurgical planning. More importantly, we used individual-level analysis, crucial for personalized treatment plans, diverging from the common practice of group-level analysis.13
Our research enhances the understanding of RSN under anesthesia by examining imaging parameters alongside the widely studied anesthesia regimen. Prior studies focused on the effect of various anesthetics on resting-state connectivity, and most used single imaging technique uniformly for all subjects in the studies. Most of the prior literature has used no accelerated rs-fMRI. However, a large sample study of healthy volunteers was reported to use accelerated rs-fMRI.28 Contrary to our hypothesis, decreased SNR in each BOLD volume of an accelerated scan did not compromise rs-fMRI, consistent with awake rs-fMRI literature.11,29 We observed no quality compromise with short scans, analyzing scans as short at 5 minutes without acceleration and 6 minutes with acceleration. The previous studies in awake subjects have shown that increasing scan length improves reliability of rs-fMRI.30,31 A recent study with anesthetized subjects further revealed that optimal scan length under anesthesia is shorter compared with awake scan because the rs-fMRI metrics show less variability under anesthesia compared with awake scan.17 It is important to note that these studies assessed the connectivity metrics and signal driven measures rather than visibility of RSN that we have evaluated in current study. Therefore, it is possible that the clinical question of visualizing RSN can be answered with shorter scans than in prior research if the scan is performed under anesthesia. These findings have potential implications for reducing scan length under anesthesia.
Sevoflurane is associated with decreased RSN robustness likely due to its established role in decreasing connectivity of frontoparietal and thalamocortical networks.14,15 Suppression of motor network has also been reported. Peltier et al32 evaluated motor network activation by using rs-fMRI in 6 volunteers and observed that, compared with awake state, functional connectivity maps were smaller under sevoflurane anesthesia. In a retrospective study of passive task-based fMRI in 100 patients, no significant effect of anesthetic choice was seen in language activation, consistent with our finding.3 The differential effect of sevoflurane on motor networks, as opposed to language networks, may arise from susceptibility of the frontal lobe, which houses the motor network, to anesthesia as opposed to resilience of the temporal lobe, which houses most of the language network. This phenomenon is well documented in the literature, including a recent work in pediatric patients with epilepsy who underwent sedated fMRI with passive tasks, where frontal language activation under sedation was weak, whereas temporal activation was more robust.33,34 We believe the gravity of motor network compromise in our data resulted in compromised combined motor-language networks as the language network was not compromised. Successful use of halogenated anesthetics has been reported in clinical settings. In a feasibility study and by using group level analysis, Warren et al13 identified default-mode, sensorimotor, and frontoparietal networks in 11 patients with epilepsy who underwent rs-fMRI with low-dose isoflurane combined with remifentanil anesthesia. In another study of 13 adults who received sevoflurane for rs-fMRI, most RSNs were reported detectable.23
It is unclear however why a similar effect was not seen with propofol, which also decreases the connectivity of frontoparietal and thalamocortical networks in a large body of literature.15,35,36 One potential explanation is the additional motion induced by administration of inhalation agents, such as sevoflurane and N2O, compared with intravenous medications such as propofol. We used motion regression in a preprocessing step and the residual motion parameters were negligible in our entire study population and did not differ between the group who received inhalation agents and the group who did not. Furthermore, the fact that the effect is only seen with sevoflurane and not N2O decreases the possibility of motion influencing the result.
Sevoflurane dosage is a critical factor affecting RSN connectivity, with higher doses leading to greater reduction in connectivity. For instance, doubling the sevoflurane dose from 1%–2% resulted in a 20% additional reduction in functional connectivity map sizes.32 While we lack precise records of sevoflurane concentration during rs-fMRI acquisition, we posit that our subjects did not receive high doses. This is based on literature indicating lower order sensory networks, like the auditory network, are maintained only up to moderate sevoflurane levels, consistent with our findings of preserved temporal activation.34,37 Additionally, varied anesthesiologists’ preferences and absence of an institutional dosage consensus suggest low likelihood of systematic administration of high doses. Our methodology accounts for potential synergistic effects by creating individual variables for commonly used anesthetics (sevoflurane, propofol, and N2O) with the caveat that only additive effects were evaluated.
Our findings should be interpreted within the study’s design and limitations, including its retrospective nature and specific patient population. The sample size was limited to 69 available subjects, but post hoc power analyses demonstrated sufficient power. The largest study with comparable design consisted of 13 subjects.13 Studies with comparable size only evaluated the resting-state functional connectivity metrics under a single uniform anesthesia regimen and image protocol.28 Other previous large studies evaluated passive task-based fMRI rather than rs-fMRI.3,38 Our study is limited by its retrospective nature, where the blood levels or mean alveolar concentration of anesthetics were not recorded and only multiband factor 5 was used. The latter may be a minor limitation in the clinic where common acceleration factors range between 2–5. Given the diverse pathology in our population, it was not feasible to group the structural abnormalities into distinct variables. The type and extent of structural abnormalities, along with treatments and medications, especially antiepileptic drugs, could influence the quality of rs-fMRI and were not controlled for in our analysis. Future studies should stratify participants by abnormality type and severity and conduct controlled studies to isolate treatment and medication effects. Potential image quality improvements from removing sevoflurane should also be explored. Future studies should include sensitivity analysis to identify a generalizable and optimal Dice coefficient cutoff for grouping the rs-fMRI data.
CONCLUSIONS
We did not observe any effect of acceleration or duration of fMRI on the quality of RSNs. With the goal of reducing scan time under anesthesia, future studies should further evaluate sufficiency of our fastest scan parameters (6 minutes with acceleration or 5 minutes without acceleration) in demonstrating clinically relevant RSNs. We observed association of sevoflurane with weaker RSNs. This association was particularly observed in the motor network but not in the language network and underscores the need for meticulous selection of anesthetic agents in fMRI protocols. Specifically, until future prospective studies can confirm our findings, it might be prudent to avoid sevoflurane if motor network mapping is required. Future studies should evaluate the effect of various dosages of sevoflurane.
Footnotes
Elmira Hassanzadeh is supported with RSNA 2022 Silver Anniversary Campaign Pacesetters Research Fellow Grant RF2203. Simon K. Warfield is supported in part by NIH awards R01 EB019483 and R01 NS124212.
Elmira Hassanzadeh and Alyssa Ailion contributed equally to this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received June 23, 2024.
- Accepted after revision July 31, 2024.
- © 2025 by American Journal of Neuroradiology