Abstract
BACKGROUND: Neoplasms derived from the sinonasal epithelium are a rare finding in the temporal bone, and their origins are controversial.
PURPOSE: To review the characteristics of sinonasal epithelial (previously known as Schneiderian) tumors occurring in the temporal bone.
DATA SOURCE: This was a 2-center case series and systematic review of MEDLINE, EMBASE, and the Web of Science through May 2021.
STUDY SELECTION: Patients with clinicopathologic evidence of temporal bone involvement by neoplasms of sinonasal epithelial origin were selected, with or without a history of prior primary sinonasal epithelial tumors.
DATA ANALYSIS: Clinical, radiologic, and pathologic data were extracted.
DATA SYNTHESIS: The systematic review included 56 studies and our 8 unpublished cases, totaling 76 cases of papillomas or squamous cell carcinomas in the temporal bone. Of these, 51% occurred secondary to sinonasal tumors, and 49% occurred primarily. Secondary tumors were usually metachronous (77%), with a median delay of 1 year from sinonasal-to-temporal bone tumor diagnosis. Most cases were unilateral (90%); bilateral temporal bone involvement occurred only as secondary (“trilateral”) tumors. Unilateral secondary tumors had ipsilateral (81%) or bilateral (19%) sinonasal counterparts. Secondary tumors were more likely to be malignant (OR, 6.7, P < .001).
LIMITATIONS: The review was based on case reports and small case series, which are subject to reporting bias.
CONCLUSIONS: The observed tumor patterns support the hypothesis that the Eustachian tube facilitates the spread of sinonasal epithelium–derived neoplasms from the sinonasal cavity to the temporal bone. Transtubal spread of sinonasal epithelium–derived neoplasms should be considered among the rare causes of middle ear masses.
ABBREVIATIONS:
- HPV
- human papilloma virus
- IQR
- interquartile range
- WHO
- World Health Organization
Sinonasal papillomas are neoplasms that arise from the sinonasal tract lining, the previously eponymous so-called Schneiderian epithelium consisting of ectodermally derived ciliated respiratory epithelium.1 In some cases, these tumors may undergo malignant transformation or arise de novo from the same epithelium. In the 2017 World Health Organization (WHO) Classification of Head and Neck Tumors and the subsequent 2022 edition, the word “Schneiderian” has been replaced by “sinonasal” for locally derived papillomas and carcinomas,2,3 but inclusion of prior terminology is necessary as a reference for historical cases and sites of origin. There are 3 subtypes of sinonasal papillomas: inverted, oncocytic, and exophytic.2,3 These papillomas may harbor or progress to carcinomas with variable frequency.
Sinonasal (Schneiderian epithelium–derived) tumors may, on occasion, occur in the middle ear. Given that sinonasal (Schneiderian) mucosa is not native to the ear and, in fact, no ciliated respiratory mucosa is located in middle ear or external auditory canal, with the middle ear lined by a simple cuboidal epithelium and the outer tympanic membrane and ear canal lined by squamous epithelium, their pathogenesis has been debated by various authors for decades. The only ciliated respiratory epithelium in the vicinity of the middle ear is along the Eustachian tube, which also contains mucinous cells. A considerable number of patients with middle ear sinonasal-type tumors also had a history of tumors in the sinonasal region, whether synchronously or metachronously, and it has been postulated that the tumor seeds or spreads through the Eustachian tube, as has been shown to occur, rarely, in nasopharyngeal carcinomas.4⇓-6
We herein present the largest primary case series, to our knowledge, of patients with middle ear sinonasal-type tumors, all of whom had a history of primary sinonasal tumors, along with a review of the current literature of these tumor types, with particular emphasis on the characterization of middle ear sinonasal-type tumors.
MATERIALS AND METHODS
Current Case Series
The radiology report database and teaching files at the authors’ 2 affiliated institutions (Massachusetts Eye and Ear and the University of California, San Francisco) were searched for cases of temporal bone imaging (CT or MR imaging) with neoplastic involvement of the middle ear, the medical records of which were further reviewed for a history of sinonasal neoplasms and other clinicopathologic information. All cases found with middle ear involvement are reported. There was 1 patient in the institutional records with sinonasal-type tumors of both the middle ear and sinonasal region that were previously reported and not included here.7 The study was approved by each of the 2 local institutional review boards.
Search Strategy
A systematic review was performed and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Online Supplemental Data). A literature search was conducted on PubMed (US National Library of Medicine), EMBASE (Elsevier), and Web of Science (Clarivate Analytics) from inception through May 27, 2021. The search string consisted of a combination of the following groups of terms: papilloma or carcinoma; Schneiderian, inverted, oncocytic, or exophytic; and temporal bone, middle ear, ear, or Eustachian tube. No additional search filters were used. Additional articles were identified by backward and forward pearl growing from relevant articles, in which the reference lists and citing articles on Google Scholar (https://scholar.google.com/) were manually reviewed. The protocol was not preregistered.
Selection Process
Eligible articles were observational studies of patients with a tumor involving the middle ear that had pathology featuring Schneiderian (sinonasal) epithelium or pathology consistent with that of a concurrent or prior epithelial tumor in the sinonasal cavity in the same patient. After we removed duplicates, records were screened for the availability of full-text articles, excluding conference abstracts or posters. Two authors (N.K. and A.F.J.) independently evaluated all full-text articles for eligibility. Exclusion criteria included non-English articles, reviews and guidelines, animal studies, meeting/conference proceedings, and studies not about Schneiderian or sinonasal neoplasms in the ear.
Data Extraction
Three authors (F.D., N.K., and A.F.J.) extracted data from the eligible full-text articles. The extracted data included study design, patient demographics (age and sex), symptoms, imaging modalities, all pathologic findings/diagnoses, and laterality of involvement. Pathologic diagnoses were categorized as benign or malignant. “Severe” or “high-grade” dysplasia, a high-risk lesion, was included in the malignant group along with carcinoma in situ and invasive carcinomas. The presence, laterality, and pathologic diagnoses of synchronous or preceding sinonasal neoplasm were also recorded. In cases in which no such data were described, it was assumed to be absent. No data were obtained from study authors directly.
Quality Assessment
The level of evidence was assessed according to the Oxford Center for Evidence-Based Medicine “Levels of Evidence 1” pertaining to etiology studies.8 Specifically, cohort study designs (level 2) were a higher level than case-control studies (level 3), which, in turn, were a higher level than case series (level 4). Potential sources of bias within and across studies were qualitatively described.
Statistical Analysis
Descriptive statistics were calculated for continuous variables, while frequencies and percentages were calculated for categoric variables. Associations between categoric variables were analyzed using χ2 test. ORs with 95% CI were computed to evaluate the association between clinicoradiologic factors and tumor malignancy. A 2-tailed P value < .05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism (GraphPad Software).
RESULTS
Case Series
Patient 1.
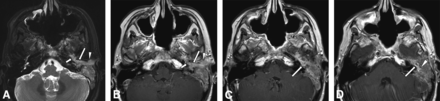
A 53-year-old woman presented with left aural fullness. She had a 30-year history of bilateral sinonasal inverted papillomas and left nasopharyngeal papillary squamous cell carcinoma 4 years prior, which was treated with definitive radiation. Imaging revealed abnormal tissue in the left middle ear, sinonasal cavity, and nasopharynx with clival invasion. Biopsy pathology from both sites showed nonkeratinizing invasive squamous cell carcinoma, possibly arising from an inverted papilloma. This case, from more than a decade prior, was a second opinion review with no tissue for additional immunohistochemistry, viral chromogenic in situ hybridization, or molecular testing. Following surgical debulking, postoperative MR imaging demonstrated the residual tumor, including in the left middle ear and mastoid, extending along the Eustachian tube (Fig 1). Despite proton beam radiation and chemotherapy, follow-up imaging showed metastatic lung and mediastinal nodal progression. Local temporal bone progression led to intracranial extension and cerebral herniation.
A 53-year-old patient with a history of bilateral sinonasal inverted papillomas and left nasopharyngeal papillary squamous cell carcinoma. Axial T2-weighted MR image (A) shows material of intermediate-to-hyperintense signal in the left posterior Eustachian tube (short arrow), middle ear (long arrow), and external auditory canal (arrowhead). Consecutive axial postcontrast T1-weighted MR images from caudal to cranial (B, C, D) show corresponding enhancement of this abnormal soft tissue in the posterior Eustachian tube (short arrow in B), middle ear (long arrow in B, C, and D), and external auditory canal (arrowhead in B), representing tumor. There is an extension to the mastoid antrum (arrowhead in D). Ear biopsy showed squamous cell carcinoma possibly arising from an inverted papilloma.
Patient 2.
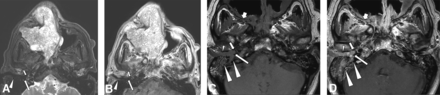
A 65-year-old man presented with right hearing loss, otorrhea, dizziness, and vertigo. He had an 8-year history of multiple, recurrent right sinonasal papillomas, inverted type, with malignant transformation to carcinoma 2 years prior (Fig 2A, -B), which was treated with partial resection, chemoradiation, and immunotherapy but locally progressed. Human papillomavirus (HPV) testing revealed no expression of high-risk HPV types. At the time of otologic presentation, imaging showed bulky enhancing tissue in the right middle ear, mastoid, and external auditory canal, with bone erosion involving the mastoid and petrous apex (Fig 2C, -D). Consistent with tumor spread to the ear, follow-up during 2 years showed tumor progression both in the sinonasal cavity and right temporal bone before the patient was transitioned to palliative care.
A 65-year-old patient with a history of recurrent, right sinonasal, inverted papilloma and carcinoma. Axial T2-weighted (A) and postcontrast T1-weighted (B) MR images show a large, bulky, lobulated septate enhancing mass in the right sinonasal region. Note the absence of abnormal soft tissue at the right Eustachian tube opening (short arrow), middle ear (long arrow), and external auditory canal (arrowhead). The patient underwent partial resection, chemoradiation, and immunotherapy. Two years later, he presented with otologic symptoms, and MR imaging was performed. Axial precontrast (C) and postcontrast (D) T1-weighted MR images show bulky enhancing soft tissue at the right Eustachian tube opening (short arrow), middle ear (long arrow), and external auditory canal (small arrowheads), with invasion into the mastoid and petrous portions of the temporal bone (large arrowheads). Also note tumor involvement in the skull base foramina such as the right pterygopalatine fossa (short broad arrows).
Patient 3.
A 60-year-old man presented with right hearing loss and otalgia for several months, failing treatments for presumed otitis externa. He had a history of right nasal cavity–based sinonasal inverted papilloma 2 years prior (Fig 3A, -B). An otologic examination found a fleshy mass in the right external auditory canal. CT and PET revealed a hypermetabolic mass centered in the right middle ear and mastoid with extensive bone erosion (Fig 3C, D, and E). Following partial resection, pathology confirmed sinonasal papilloma, inverted type, in both nasal and right ear sites. When he returned to follow-up 1.5 years later, imaging showed local tumor progression in the right temporal bone, including tumor tracking along the Eustachian tube (Fig 3F, -G). A CT 4 months later showed right level II adenopathy, and biopsy revealed a low-grade sinonasal (Schneiderian) squamous cell carcinoma. The tumor was negative for high-risk HPV, but the patient did have a mutation in p53 with additional loss of CDKN2A. Despite starting chemoradiation, thoracic metastases developed, leading to a focus on comfort care.
A 60-year-old patient with a right nasal cavity inverted papilloma. Axial precontrast (A) and postcontrast (B) T1-weighted images show the initial right sinonasal papilloma (arrow) 2 years prior. On presentation to otology, CT in soft-tissue (C) and bone (D) windows shows an expansile bulky mass in the right middle ear (long arrow in C), external auditory canal (arrowhead in C), and mastoid (large arrowhead in C) with bone destruction (arrows in D). PET (E) shows avid FDG uptake by the mass (arrow). Axial precontrast (F) and postcontrast (G) T1-weighted images show enhancing tumor along the right Eustachian tube (small arrow), in the middle ear (long arrow), and in the mastoid (large arrowhead). Pathology of the ear mass proved to be inverted papilloma.
Patient 4.
A 68-year-old man presented with left hearing loss for 2 months with 6 months of left nasal congestion and drainage. CT and MR imaging revealed a mass in the left nasal cavity extending to the left nasopharynx, abutting the Eustachian tube orifice; enhancing tissue was also present within the left middle ear. Surgical pathology review demonstrated a low-grade sinonasal carcinoma with mixed features of sinonasal (Schneiderian), salivary, and odontogenic origin. The tumor was negative for high-risk HPV and Epstein-Barr-encoded messenger RNA (by chromogenic in situ hybridization. The tumor was revealed to have an NF1 variant.
Five months later, he presented with worsening left ear hearing and otalgia. MR imaging showed persistent enhancing tissue in the left posterior Eustachian tube and middle ear. A nonpulsatile erythematous mass was present on otoscopy. A middle ear mass biopsy demonstrated low-grade sinonasal carcinoma with histologic features similar to those in the previous sinonasal tumor. The patient underwent resection and proton beam therapy and showed no recurrence with 2 years of surveillance.
Patient 5.
A 70-year-old woman presented with imbalance and bilateral hearing loss. Examination revealed a polypoid mass in the left external auditory canal. Biopsy indicated atypical squamous proliferation. CT and then MR imaging showed a soft-tissue mass at both nasal cavities, nasopharynx, and bilateral middle ear and mastoid regions, including near the Eustachian tubes. Additional biopsies of the masses in the right nasal cavity and right ear canal confirmed benign sinonasal papillomas, inverted type. No additional information regarding molecular or viral status is known about this case.
Patient 6.
A 45-year-old man presented with hearing loss. He had a history of sinonasal inverted papilloma with high-grade dysplasia, resected 3 years prior, with recurrence as sinonasal squamous cell carcinoma, which had been resected 1 year before the current presentation. Review of previously performed MR imaging showed a large sinonasal mass that extended to the nasopharynx, abutting the left Eustachian tube, and abnormal enhancement along the left Eustachian tube and in the middle ear cavity. At the latest presentation, tumor was visible in both external auditory canals; bilateral biopsies demonstrated sinonasal squamous cell carcinoma evolving from sinonasal inverted papilloma. Imaging at this time confirmed destructive enhancing soft-tissue masses in the bilateral mastoid regions invading intracranially. No additional information regarding molecular or viral status is known about this case. He subsequently developed pulmonary metastases and entered hospice care.
Patient 7.
A 70-year-old man presented with otalgia, otorrhea, trismus, facial swelling, and tooth loss. He reported a history of a “nodule” being surgically removed from his nasal cavity overseas 3 months prior, with unknown pathology. MR imaging demonstrated a destructive mass in the right maxillary sinus and nasal cavity, enhancing soft tissue in bilateral Eustachian tubes and temporal bones, and dural enhancement with frank intracranial extension. Nasal mass biopsy established a diagnosis of sinonasal squamous cell carcinoma with associated benign sinonasal inverted papilloma. Despite chemotherapy, the patient had disease progression and eventually died from the disease complicated by tumoral hemorrhage and respiratory failure.
Patient 8.
A 36-year-old man with a previous diagnosis of Bell palsy presented with progressive bilateral facial paresis, hearing loss, otalgia, otorrhea, and rhinorrhea. One month prior, tumor was identified in the bilateral ear canals, and a biopsy indicated sinonasal inverted papilloma. At our institution, examinations revealed destructive, multicystic masses in the nasal cavity, nasopharynx, and bilateral mastoid temporal bones. There was intracranial invasion, left sigmoid sinus thrombosis, and temporal lobe edema. Debridement revealed squamous cell carcinoma in the nasopharynx and sinonasal inverted papilloma with severe squamous dysplasia in the skull base. No additional information regarding molecular or viral status is known about this case. Three months later, the patient had left temporal intracerebral hemorrhage, thought to be related to either direct tumor invasion or venous thrombosis.
Systematic Review
A PRISMA flow diagram is shown in Fig 4, detailing the systematic identification, screening, and determination of inclusion or exclusion of publications. A total of 56 articles containing 68 cases were included (Online Supplemental Data).
PRISMA flow diagram showing search algorithm used for systematic review.
Bias.
All included published studies were case reports or case series up to a maximum of 4 patients. According to the Oxford Center for Evidence-Based Medicine Levels of Evidence of etiology, case series are level 4 out of 5. Case series are at risk of selection/publication bias, so unusual features or associations may be overrepresented compared with typical practice.
Patient and Tumor Site Characteristics.
The systematic review and our case series of 8 patients sums the current analysis to a total of 76 cases. There were 45 males and 31 females (ratio 1.5:1). The median age at presentation of the middle ear tumor was 53 years (range, 11–82 years; interquartile range [IQR], 44–63 years).
Of these, 37 (49%) patients had tumor involving the middle ear primarily without sinonasal involvement, while 39 (51%) patients had tumor involving the middle ear, secondary to sinonasal involvement. In no case did Schneiderian-type tumor in the middle ear precede sinonasal occurrence of tumor. Of the secondary tumor cases, the median reported time interval between the sinonasal and ear tumor diagnosis was 1 year (range, 0–40 years; IQR, 0.5–4 years); cases were considered synchronous (within 6 months) with the sinonasal tumor in 9/39 (23%) and metachronous (at least 6 months) in 30/39 (77%).
Of the cases that reported the laterality of tympanomastoid involvement, most cases were unilateral (65/72; 90%). Of the secondary tumor cases that reported a laterality of sinonasal involvement, most cases were unilateral (31/38; 82%). All 7 cases of sinonasal-type (Schneiderian) tumors presenting in the bilateral tympanomastoid cavities were secondary to a sinonasal tumor, ie, “trilateral.” Unilateral secondary sinonasal-type tumors of the ear had ipsilateral (25/31; 81%) or bilateral (6/31; 19%) sinonasal counterparts; there were no cases of unilateral tympanomastoid and contralateral unilateral sinonasal involvement.
Clinical Presentation.
The 76 patients with sinonasal-type tumors in the middle ear most commonly presented with the following: hearing loss (67%), otorrhea (47%), aural fullness (18%), otalgia (17%), and tinnitus or pulsatile tinnitus (16%), among others (Table 1). When imaging was reported, most patients underwent both CT and MR imaging (34/61; 56%; including all 8 cases in our series) or CT alone (25/61; 41%).
Summary of patient characteristics in systematic review and case series of temporal bone Schneiderian neoplasmsa
Contingency table on the association of temporal bone Schneiderian tumors that are primary (isolated) or secondary (synchronous or metachronous to a similar sinonasal tumor) and pathology that is malignant/high-risk (carcinoma, carcinoma in situ, or papilloma with severe/high-grade dysplasia) or benign (papilloma without severe/high-grade dysplasia)
Histopathologic Characteristics.
The histopathology of the middle ear tumors on initial diagnosis was benign in 65% (48/74) and malignant in 35% (26/74); the ear component of 2 cases from our series was diagnosed as malignant on clinicoradiologic grounds. Primary (isolated) tumors were predominantly benign (31/37; 84%), while secondary sinonasal-type tumors of the ear were often malignant (22/39; 54%) (Table 2). The association of secondary tumors with malignancy was statistically significant (OR, 6.7; 95% CI, 2.3–19.1; χ2, P < .001).
Of the secondary tumor cases, the sinonasal tumor histopathology closest in time to the ear tumor diagnosis was benign in 62% (24/39) and malignant in 38% (15/39). Tumors in the middle ear and the sinonasal region were always of the same histologic lineage (sinonasal papilloma/carcinoma).
Testing for HPV and other viral or molecular markers was often not performed or reported. In the 15 cases in which findings of HPV were negative, most tumors were benign (11/15; 73%). In the 12 cases in which findings of HPV were positive, most were malignant (7/12; 58%).
We examined the role of the Eustachian tube in disease involvement and spread. Among the 39 patients with secondary tumors, 9 (23%) were reported to be positive for Eustachian tube tumor involvement by biopsy/surgical resection histology or by intraoperative visual examination, 16 (41%) had imaging studies showing enhancing soft tissue consistent with tumor along the Eustachian tube, and 3 (8%) were presumed to be involved due to clinical presentation or proximity of lesions. In 6 case reports (15%), the Eustachian tube was thought not to be involved either by CT, visual inspection during surgery, biopsies taken along the Eustachian tube, or not otherwise specified. The Eustachian tube was not mentioned in 5 cases.
DISCUSSION
We present a comprehensive systematic review that incorporates all reported cases in the English literature on sinonasal-type (Schneiderian) tumors involving the middle ear, with or without antecedent or concurrent tumors of the same histopathologic lineage in the sinonasal region. We also present a robust cohort of previously unreported cases in patients with concomitant middle ear and sinonasal tumors.
Because the sinonasal (Schneiderian) epithelium is not native to the temporal bone, hypothesized mechanisms for tumors of sinonasal (Schneiderian) origin developing in the middle ear include the following: 1) direct spread via the Eustachian tube, akin to the manner in which nasopharyngeal carcinoma has been shown to spread to the middle ear;4⇓-6 2) metaplasia of the middle ear mucosa to Schneiderian mucosa, perhaps as a result of chronic inflammation (though this may be of doubtful validity because inflammation-induced metaplasia does not convert the simple cuboidal epithelium of the middle ear canal into ciliated respiratory [Schneiderian] mucosa); and 3) anomalous embryonic migration of Schneiderian-type ciliated respiratory mucosa. The Eustachian tube is a physical connection between the 2 sites, and if nasopharyngeal carcinoma can extend via the Eustachian tube to the middle ear,4⇓-6 then sinonasal tumor spreading along the sinonasal and pharyngeal mucosa could well continue along the Eustachian tube in the same manner.
Direct tumor spread from the sinonasal region to the middle ear is a well-supported mechanism.9 More than one-half of the cases reviewed here had a synchronous or metachronous prior diagnosis of sinonasal tumor of similar histologic lineage (sinonasal mucosa origin). All those tumors presented concurrently with or subsequent to the sinonasal tumor diagnosis, never preceding it, and were more likely malignant. The involved ear was never contralateral to the side of the sinonasal tumor, only ipsilateral or bilateral. All 8 patients in our series and a number of previously published cases showed involvement of the Eustachian tube on imaging (characterized by soft tissue isodense/isointense to tumor along the Eustachian tube course). These observations support the postulation that sinonasal tumors may spread through the Eustachian tube.
Squamous tumors of different sites may have variable histologic morphologies. Some squamous epithelium is keratinizing or nonkeratinizing, some lesions have viral cytopathic changes, and some locations may boast squamous epithelium of mixed morphology. When tumors spread to unusual sites and have multiple possible primary sites, identifying the tumor origin may require more in-depth tissue evaluation, particularly involving site/tissue-specific genetic signatures, such as epidermal growth factor receptor or HPV-associated anomalies in sinonasal (Schneiderian) tumors.10
We propose that in patients with sinonasal-type tumors, clinical and imaging surveillance should include otologic examination. Temporal bone involvement may currently be underdetected. Radiologists should be aware of the need to scrutinize the Eustachian tube and middle ear, whether on CT, MR imaging, or potentially PET. While middle ear effusions are common after treatment of sinonasal tumors,11 the alternative possibility of tumor spread to the middle ear should be considered when there is solid, enhancing tissue in the middle ear similar to the original sinonasal tumor. Given the aggressive nature of malignant sinonasal tumors and the high rate of malignancy when found in the middle ear, it behooves clinicians to scrutinize the Eustachian tube region and have a low threshold for otology consultation and consideration of biopsy. Furthermore, the concept of the primary origin of these tumor types in the middle ear, without a thorough examination of prior history and imaging, should warrant revisitation, given that all the cases in this series showed concurrent or prior sinonasal tumor. Most important, prompt detection of tumor spread may influence management such as radiation therapy target delineation.
This review relies on case series and case reports. The reporting of features may be selective and inconsistent across time because additional testing modalities and genetic signatures have evolved with their increased availabilities. This inconsistency limits the generalizability of observed associations. Also, given that these are historical cases that have accumulated during longer than the past decade, some testing modalities were not available for some cases, particularly some of the molecular testing for consult cases, and molecular data are incomplete.
CONCLUSIONS
The observed tumor patterns in this systematic review and our case series support the hypothesis that the Eustachian tube may facilitate the spread of sinonasal (formerly Schneiderian) neoplasms from the sinonasal cavity to the temporal bone. Transtubal spread of sinonasal-origin neoplasms should be considered among the rare causes of middle ear masses. For the subset of this tumor type reported by others to appear primarily in the middle ear, a thorough review of the patient’s history, particularly with respect to any prior sinonasal surgery, for any reason, is essential. Clinicians and radiologists should be diligent when considering middle ear disease in patients with sinonasal neoplasms to avoid neglecting tumor extension to the middle ear from a sinonasal source, ensuring prompt and appropriate treatment considerations of all involved sites. Recent progress in molecular classification of these tumors may also help in discerning tumor type/origin.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received October 18, 2023.
- Accepted after revision December 7, 2023.
- © 2024 by American Journal of Neuroradiology