Abstract
BACKGROUND AND PURPOSE: The T2-weighted MR imaging total lesion volume and Expanded Disability Status Scale (EDSS) score are two common measures of relapsing-remitting multiple sclerosis disability and pathologic abnormality. Because the whole-brain N-acetylaspartate concentration is considered to be a new marker of the disease burden, the purpose of this study was to evaluate the relationship among these three measures.
METHODS: The whole-brain N-acetylaspartate concentration and T2-weighted lesion volume were quantified by using MR imaging and proton MR spectroscopy in 49 patients with relapsing-remitting multiple sclerosis (36 female and 13 male patients; average age, 39 years; age range, 24–55 years; average EDSS score, 2; range of EDSS scores, 0–6). Correlations among whole-brain N-acetylaspartate concentrations, T2-weighted lesion volumes, and EDSS scores were obtained.
RESULTS: No correlation was found between whole-brain N-acetylaspartate levels and either T2-weighted lesion volumes or EDSS scores. A weak correlation was found between the EDSS scores and T2-weighted lesion volumes (P = .043, rs = 0.292).
CONCLUSION: Despite the lack of correlation between whole-brain N-acetylaspartate concentration and the clinical disability reflected in the EDSS score, only the former evaluates the global neuronal cell disease in the entire brain, including those lesions that are occult to conventional imaging techniques.
Dual-echo proton density–weighted T2-weighted MR imaging is a common radiologic tool for the investigation of multiple sclerosis. However, T2-weighted MR imaging has recognized limitations in depicting the full extent of pathologic abnormality at the ventricular boundaries because of poor contrast resolution between lesion and CSF signal, and it fails to depict occult pathologic abnormality in normal-appearing white matter (1). Furthermore, T2 hyperintensity is nonspecific and represents a wide spectrum of potential pathologic abnormalities (eg, inflammation, demyelination, edema, gliosis, and axonal loss) (2, 3). Poor specificity for gross pathologic abnormality and lack of sensitivity to subresolution pathologic abnormality probably contribute to the poor correlation between T2-weighted lesion volume and the standard neurologic measure: Kurtzke’s Expanded Disability Status Scale (EDSS) score (4–8).
To address these concerns, proton MR spectroscopy has been applied to augment T2-weighted MR imaging. In vivo proton MR spectroscopy can be used to noninvasively quantify brain metabolites, such as N-acetylaspartate (NAA), choline, and creatine (9). NAA, an amino acid derivative, is present almost exclusively in mature neurons (10). Its level has been reported to decrease in both acute and chronic multiple sclerotic plaques (11–15) and in normal-appearing white matter (16–19); this change makes its reduction a surrogate marker of axonal damage or loss (11). Previous MR spectroscopy studies, however, used mostly single-voxel techniques, which quantify only a small (<100 cm3) volume of the brain (11–19) and fail to reflect the full extent of the damage. The introduction of whole-brain NAA quantification has enabled the evaluation of the NAA level in more than 90% of the brain (ie, in more than 90% of the lesions, the normal-appearing white matter, and the gray matter) (20–22). The unequivocal connection between NAA deficits and axonal loss in cases of multiple sclerosis has only recently been established by Bjartmar et al (23), who used immunopathologic and immunocytochemical findings in postmortem spinal cord samples obtained from lesions and normal-appearing white matter of patients and from white matter in age-matched control subjects. Lower axonal density and proportionally lower NAA-unit volume are both correlated with the neurologic impairment in these patients (23). Previously, Trapp et al (24) used similar methods to show that axonal loss in acute and chronic cerebral multiple sclerotic lesions is also correlated with NAA reduction (24). Therefore, whole-brain NAA deficit is a direct, noninvasive measure of total axonal loss, which, in turn, reflects the global pathologic load of the disease (12, 20, 25).
For the present study, we correlated whole-brain NAA levels with T2-weighted lesion volumes and EDSS scores in a cohort of patients with relapsing-remitting multiple sclerosis. Whole-brain NAA levels quantifies axonal damage exclusively in the brain. In contrast, the EDSS score, which is an aggregate measure of ambulation, sensory, visual, brainstem, cerebellar, pyramidal, bowel, bladder, and cerebral function, is weighted for motor performance (ie, it mainly reflects spinal cord pathologic abnormality). Finally, the cerebral lesion volume on T2-weighted images is insensitive to both occult pathologic abnormality in the normal-appearing white matter and spinal damage. Consequently, our working hypothesis was that both the lesion volume or T2-weighted image and EDSS score would correlate poorly with whole-brain NAA. Furthermore, because NAA concentration has shown stronger correlation with overall clinical disability than either the lesion volume or EDSS score (26), whole-brain NAA concentration level could be a more relevant measure of the effective burden of the pathologic abnormality (12).
Methods
Patients
Forty-nine patients (36 female and 13 male patients) with clinically definite relapsing-remitting multiple sclerosis according to the Poser criteria (27) were recruited for this study. The mean patient age was 39 years (age range, 24–55 years), and the mean disease duration, measured from the time of clinical onset, was 8.7 years (range of disease duration, 0.8–23.5 years). Patients’ individual disabilities were classified according to EDSS scores (26). Ranging from 0 (normal function) to 10 (death) in steps of 0.5, an EDSS score of less than 3 indicated minimal disability, whereas an EDSS score of greater than 5.0 entailed serious physical and mental limitations. The mean EDSS score in this study’s cohort, determined within 2 months of the imaging protocol, was 2 (range of EDSS scores, 0–6). Eleven of the participants were receiving immunomodulatory medications during these measurements; however, none had relapse during that time. All participants were briefed on all procedures, and all participants provided written informed consent. Institutional review board approval was obtained.
MR Imaging
All MR imaging of the brain was performed with a clinical 1.5-T imager with a standard transmitter-receiver head coil. The protocol comprised a proton density–weighted and T2-weighted fast spin-echo sequence (2500/16 and 80 [TR/TE]; matrix, 192 × 256; field of view, 220 mm; contiguous section thickness, 3 mm).
Proton MR Spectroscopy: Whole-Brain NAA Quantification
The amount of NAA in the entire brain superior to the foramen magnum, QNAA, was obtained by using a 4-T whole-body imager with its standard head coil. Our high-order (third) autoshimming procedure yielded a consistent 30 Hz ± 5 full-width-at-half-maximum whole-head water line width. Non-localizing, non-echo, proton MR spectroscopy was then performed to obtain the whole-head NAA signal, as described previously (21). The entire procedure required approximately 25 minutes.
Absolute quantification of the NAA, which renders the method independent of the specific MR instrument brand, its magnetic field strength, and the performance site, was conducted against a reference 3-L sphere of 15 mmol NAA in water. Participant and phantom NAA peak areas, SS and SP, were numerically integrated, and QNAA was calculated as follows (21): 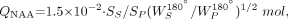 1)
1)
where WP180° and WS180° are the transmitter power into 50 Ω required for a nonselective 1-ms 180° inversion pulse on the phantom and participant respectively, reflecting the system’s sensitivity. To compensate for the natural variability of brain sizes, QNAA was divided by the participant’s brain volume, VB, obtained from high-resolution MR imaging at 1.5 T, as described in the next section. A previous multiple-serial-measurements study to quantify the reproducibility of the whole-brain NAA method has shown the intra- and interindividual variability to be less than 5% and 6%, respectively (21).
Postprocessing
The images were transferred to a workstation and segmented with the 3DVIEWNIX software package described by Udupa and Samarasekera (28) and Udupa et al (29). Specifically, based on several manually preselected intensity points in the CSF, gray matter, and white matter, the software uses the fuzzy connectedness principle to create a brain mask. The mask is then manually corrected to remove the skull and non-brain soft tissue, add missing brain parts, and remove the CSF. Finally, VB is obtained by counting the remaining pixels within the corrected mask. This method has reproducibility of greater than 99% (30).
T2-weighted lesion volumes were also calculated with 3DVIEWNIX. The precision and accuracy of this aspect of the software have been validated and reported elsewhere (29, 31). Specifically, based on the principle of fuzzy connectedness, 3DVIEWNIX enables the user to detect lesions and calculate their volumes semiautomatically (29). The operator has only to accept or reject computer-delineated lesions while maintaining the ability to manually edit under- or overrepresented lesions.
Statistics
Comparisons between whole-brain NAA concentration, T2-weighted lesion volume, and EDSS were assessed with nonparametric Spearman correlation coefficient analysis. All differences were considered significant at a P value of .05 or less. Because only 11 of the 49 patients were receiving immunomodulatory treatment, of different types and lengths (either in absolute terms or as a percent of disease duration) during the study, this factor was excluded from the analyses.
Results
The mean whole-brain NAA concentration level for all 49 participants was 10.86 mM (range, 5.14–15.82 mM). The mean lesion volume was 6.86 cm3 (range, 0.18–50.65 cm3), and the mean brain volume was 1023 cm3 (range, 768–1217 cm3). No significant correlation was found between whole-brain NAA concentration and lesion volume (P = .762) or between whole-brain NAA concentration and EDSS score (P = .985). The lesion volume was weakly correlated with the EDSS score (P = .043, r = 0.292). Patient age was also compared with these criteria and was shown to be independent of all measures. Therefore, any statistical relationship found was probably due to pathologic abnormality and not to the aging process. These results are summarized in the Table.
Comparisons among whole-brain NAA, EDSS, lesion volume on T2-weighted images, and age
Discussion
Common clinical assessments of multiple sclerosis (eg, EDSS scores) are inconsistent with the radiologic standard in this disease: T2-weighted MR imaging findings (5, 6). Although it can depict the gross pathologic abnormality, T2-weighted MR imaging lacks specificity for the type of damage and its extent. In addition, it is insensitive to occult disease in normal-appearing white matter. Because lesion volume on T2-weighted images is rarely greater than >5% of the brain volume, such microscopic disease potentially represents a far greater fraction of the overall burden (6).
This lack of specificity and sensitivity motivated the development of other radiologic measures to reflect specific pathologic forms in the entire brain. These include global atrophy (32, 33); hypointense lesion volume on T1-weighted images, which is assumed to represent areas of matrix destruction and axonal loss (34); and whole-brain NAA concentration (20). Although all three represent permanent damage in cases of multiple sclerosis, only the whole-brain NAA concentration reflects global neuronal degeneration in all lesions, normal-appearing white matter, and gray matter and may, therefore, be considered to be a powerful marker of the disease load (20). Although the specific function of NAA is unknown, it is thought to be present almost exclusively in neurons and their processes (10, 35). It should be noted, however, that oligodendrocyte type 2 astrocyte progenitor cells (36) and mature oligodendrocytes (37) also express NAA and may reduce its specificity to reflect neuronal damage. Nevertheless, this has been described only in vitro, and its relevance to the in vivo situation is not certain. In addition, the population of oligodendrocyte precursor cells in multiple sclerotic lesions is relatively dormant, and their potential NAA level is considered minimal (38). Consequently, NAA concentration is potentially a powerful marker of neuronal integrity.
Initial work (11) suggests that the decrease of NAA was a measure of irreversible axonal loss. However, transient and reversible decreases in NAA during the acute phase of the disease were reported more recently; this finding supports the possibility of metabolic axonal dysfunction or reversible volume loss (12, 13). Using immunohistochemistrys, Trapp et al (24) showed that such NAA decreases could be explained by axonal transection secondary to inflammatory demyelination, as well as by marked alteration of axonal caliber. To differentiate all these possibilities, Bjartmar et al (23) compared postmortem axonal density in the spinal cord of patients with chronic multiple sclerosis with NAA levels assessed with high performance liquid chromatography. They showed that axonal loss is a major cause of NAA decrease in the chronic stages of multiple sclerosis; this result strongly supports the need for a method of measuring these changes in vivo.
Nevertheless, despite recent reports that correlated regional NAA concentrations with EDSS scores (39–41), no such correspondence was found with whole-brain NAA. Two explanations are possible for this discrepancy. First, the NAA level was frequently expressed in the literature as an NAA-creatine ratio, in which the creatine levels is assumed to be stable even in cells with chronic pathologic abnormality. However, Suhy et al (42) recently reported variations of the absolute creatine level in cases of multiple sclerosis, which was attributed to gliosis, suggesting that the NAA-creatine ratio should be used with caution. Other studies used a 2-cm-thick axial volume of interest placed on a centrally situated section of the brain along the craniocaudal axis (39–41). This covered only a fraction of the potentially damaged volume and forced a difficult-to-verify assumption regarding its uniformity with the rest of the CNS. In contrast, whole-brain NAA yields absolute NAA concentrations in all infra- and supratentorial lesions, normal-appearing white matter, and gray matter (21).
The second possible explanation for the lack of whole-brain NAA-EDSS correlation may be the limited focus of the latter, its variability among observers (43, 44), and its heavy weighting toward motor dysfunction (ie, spinal pathologic abnormality undetected by whole-brain NAA). The EDSS score also does not fully account for memory, fatigue, or cognitive functions, all essential components of quality of life for patients with multiple sclerosis (45, 46). These limitations motivate the search for an alternative novel clinical outcome measure, such as the Multiple Sclerosis Functional Composite measure developed by the National MS Society’s Clinical Outcome Assessment Task Force (47).
The EDSS scores and lesion volumes on T2-weighted images in this cohort were significantly correlated (P = .043), and they were similar to correlations previously reported by other groups (4–8). However, as in the other studies, the correlation was weak, accounting for less than 10% of the variability of the data (rs = 0.292). Furthermore, individual lesion location is rarely considered in the context of the total T2-weighted lesion volume, and this study was no exception. Therefore, it is possible that random focal pathologic abnormalities in motor-eloquent brain regions also influence EDSS scores, and, thus, they could be responsible for the observed weak correlation, both in this study and in previous studies. The lack of correlation between whole-brain NAA values and lesion volume is not completely unexpected. First, the lesion volume contained all hyperintensities, including those stemming from the transient pathologic abnormalities described in the introduction of this article (2, 3). Some of these resolve in time and do not represent permanent damage. Second, only actual lesions are reported; occult disease in the normal-appearing white matter was not accounted for (13, 48, 49). As a result, lesion volume on T2-weighted images rarely exceeds 5% of the total-brain parenchymal volume (6), which is insufficient to explain the variations in the degree of disability experienced by people with similar lesion loads (46). In contrast, whole-brain NAA values are used to estimate neuronal cell damage in the entire brain, irrespective of whether the tissue is lesional, contains subresolution pathologic abnormality, is normal white matter, or is gray matter. Therefore, the whole-brain NAA concentration is a more comprehensive estimate of accumulated disease burden.
Conclusion
No correlation was found between whole-brain NAA concentration levels and T2-weighted lesion volumes or EDDS scores in this study population. This result should not be surprising, because the volumetric and grading underestimations of T2-weighted lesion volumes and the only partial weighting of cerebrum pathologic abnormalities by the EDSS scores are remedied by the use of whole-brain NAA levels, which can be used to evaluate neuronal integrity in more than 90% of the brain, including lesions, white matter, and gray matter. Consequently, we suggest that the whole-brain NAA concentrating provides the most comprehensive and specific estimate of neuronal and axonal dysfunction in the brain.
Acknowledgments
The authors thank the study’s patient coordinator, Lois Mannon, RT, for her invaluable efforts.
Footnotes
This work was supported by National Institutes of Health grants NS33385, NS37739, and NS29029 and by a grant from the French Society of Radiology.
References
- Received June 6, 2001.
- Accepted after revision August 15, 2001.
- Copyright © American Society of Neuroradiology












