Abstract
Summary: The intravenous administration of abciximab can be used as an adjuvant therapy to facilitate thrombolysis for acute cerebrovascular occlusion. However, to our knowledge, the intraaterial administration of abciximab has not been reported. We recently treated three patients with acute thrombosis of the cerebral arteries by using an intraarterial infusion of urokinase and abciximab. Even with small doses, we achieved rapid and complete recanalization without complications. We believe that the intraarterial infusion of abciximab may be promising for effective and safe recanalization of acute thrombotic occlusion of cerebral arteries.
The most important objective in the treatment of acute cerebral arterial occlusion is a rapid and safe restoration of the blood flow. However, neurointerventionalists commonly encounter cerebrovascular occlusions resistant to thrombolytic agents. Recent studies indicate that the key factor in this resistance is an explosive thrombin generation, and adjuvant use of potent antiplatelet agents, such as abciximab, can facilitate thrombolysis by blocking this process (1–4). However, the systemic use of abciximab can be dangerous in cerebrovascular thrombotic lesions. The risk of cerebral hemorrhage after acute occlusion of cerebral arteries can be increased with the use of thrombolytic or antiplatelet agents. In addition, thromboembolic complications can develop during cerebrovascular interventions with high hemorrhagic risks, such as embolization of ruptured intracranial aneurysms or ruptured arteriovenous malformations. Therefore, the reduction of hemorrhagic risk, as well as the rapid and successful reopening of vessels, is important, especially in the treatment of acute cerebrovascular thromboembolic lesions.
We recently treated three patients with acute thrombosis of the cerebral artery. With an intraarterial infusion of urokinase and abciximab, we achieved rapid and complete recanalization without complications. In this report, we present our cases and describe the advantages of the intraarterial use of abciximab in cerebrovascular interventions.
Case Reports
Case 1
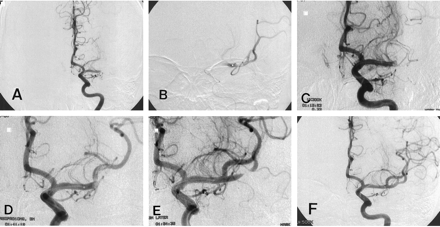
A 26-year-old woman was admitted with right-sided hemiplegia and motor aphasia that had developed suddenly 5 hours before admission. The results of nonenhanced CT of the brain were normal. A diagnosis of acute occlusion of the left carotid or middle cerebral artery was made, and angiography was performed with local anesthesia. Systemic heparinization (5000-U bolus injection and then continuous infusion at a rate of 1000 U/h) was administered during the procedure. A selective injection of the left carotid artery showed occlusion of the middle cerebral artery (Fig 1A). A 6F guide catheter was placed in the cervical internal carotid artery, and urokinase was slowly infused into the clot by means of a coaxially inserted microcatheter. After the manual infusion of 100,000 U of urokinase, partial opening of the M1 and M2 segments was visualized (Fig 1B). Additional urokinase was slowly infused, but angiograms obtained immediately after the infusion of 200,000, 300,000, and 400,000 U of urokinase paradoxically showed an aggravation of the occlusion (Fig 1C). The microcatheter was slightly withdrawn and positioned proximal to the occlusion, and 10 mg (5 mL) of undiluted abciximab was administered through the catheter over 5 minutes. Five minutes after the administration, angiography was performed and revealed partial recanalization of the superior division of the middle cerebral artery (Fig 1D). Remaining thrombi within the superior division were also observed. Repeat angiography was performed another 5 minutes later and showed a marked improvement in patency (Fig 1E). Only focal occlusion in the inferior division remained. At that time, the patient fully recovered. Hemiplegia and motor aphasia completely disappeared. The microcatheter was advanced into the remaining thrombus, and an additional 100,000 U of urokinase was injected. Repeat angiography showed further recanalization (Fig 1F). Although one large branch of the inferior division was still occluded, additional drugs were not administered because, 8 hours after the first appearance of the symptoms, angiograms showed no missing artery owing to abundant collaterals, and the patient fully recovered. Heparin was administered for 2 days after the procedure. The patient was discharged without neurologic deficit after a stay of 10 days. Laboratory studies for coagulation abnormalities, connective tissue diseases, and cardiac evaluation did not reveal any specific abnormalities. Aspirin and clopidogrel were administered for 3 months. Four months later, the patient had no neurologic deficit.
Case 1. Angiograms show an acute middle cerebral artery occlusion treated with intraarterially administered urokinase and abciximab.
A, Initial angiogram shows occlusion of the left M1 segment.
B, After the intraarterial infusion of urokinase (100,000 U), the artery is partially opened.
C, Angiogram obtained after the intraarterial infusion of urokinase (300,000 U) shows aggravation of thrombotic occlusion of the M1 segment.
D, Angiogram obtained 5 minutes after intraarterial infusion of 10 mg of abciximab shows a partial recanalization. Remaining thrombi are seen within the superior division of the middle cerebral artery.
E, Angiogram obtained another 5 minutes later shows more advanced recanalization.
F, Final angiogram shows further recanalization. Although one branch of the inferior division of the middle cerebral artery is still occluded, in lateral projection, there was no missing artery owing to well-developed collaterals (not shown).
Case 2
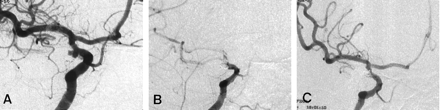
A 52-year-old woman was admitted for the treatment of an incidentally found aneurysm. Angiography revealed a bilobulating aneurysm of the right posterior communicating artery (Fig 2A). A mild luminal irregularity, probably due to atherosclerosis, was observed in the internal carotid artery. A decision was made to perform embolization with Guglielmi detachable coils. With the patient under general anesthesia, a 6F guide catheter was positioned in the right internal carotid artery, and the aneurysm was selected with a microcatheter. Systemic heparin was administered (a 3000-U bolus and then continuous infusion at a rate of 1000 U/h). Initially, obliteration of the two lobes with one large coil was tried, but it failed; therefore, separate packing of the lobes was tried. While the first coil was being placed in the superior lobe, the aneurysm ruptured. This rupture was induced by a protrusion of a small coil loop outside the aneurysm wall. However, as the coil was further positioned, bleeding soon stopped. After obliteration of the superior lobe, the catheter was pulled back gently and inserted into the inferior lobe. A coil (Guglielmi detachable coil–10, 2 mm × 4 cm, soft, stretch resistant) was placed, but during deployment, the coil was fractured. Angiography performed after the fracture revealed a thrombotic occlusion of the internal carotid artery (Fig 2B). The microcatheter was withdrawn from the aneurysm, and the thrombus was laced with 160,000 U of urokinase; the tip of the catheter was placed proximally, internally, and distally to the clot. However, the occlusion was unaffected. For fear of potential rebleeding from the superior lobe, which had ruptured approximately 20 minutes before, additional urokinase was not used. Instead, 5 mg (2.5 mL) of abciximab was infused through the guide catheter over a 1-minute period. Angiography performed 5 minutes later showed obvious recanalization though a luminal narrowing in the parent artery due to a protruding coil mass that remained (Fig 2C). The aneurysm was not completely occluded, but further coil packing was not attempted. The patient fully recovered from anesthesia and had no neurologic deficit. Heparinization was continued for 2 days after the procedure. Aspirin and clopidogrel were administered. The patient’s neurologic status remained normal during 5 months of follow-up.
Case 2. Angiograms show a thrombotic complication that occurred during embolization with Guglielmi detachable coils; this was successfully treated with intraarterial abciximab.
A, Angiogram shows a bilobulated posterior communicating artery aneurysm.
B, Angiogram obtained after detachment of the last coil shows thrombotic occlusion of the parent artery.
C, Angiogram obtained 5 minutes after intraarterial infusion of abciximab (5 mg) through a guide catheter shows recanalization, although there remains a luminal narrowing of the parent artery due to the protruding coil mass.
Case 3
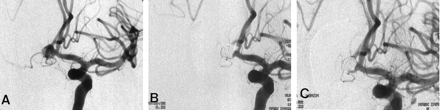
A 25-year-old man visited the emergency department because of a sudden severe headache. The patient was conscious, and his neurologic findings were normal. CT of the brain revealed a diffuse subarachnoid hemorrhage, and cerebral angiography revealed an anterior communicating artery aneurysm (Fig 3A). The right A1 segment was aplastic. A decision was made to administer endovascular treatment. With the patient under general anesthesia and with the administration of heparin (a 3000-U bolus and then continuous infusion at a rate of 1000 U/h), a 6F guide catheter was positioned in the left internal carotid artery. With use of a conventional coil embolization technique, the aneurysm was completely obliterated with five Guglielmi detachable coil–10s. However, angiograms obtained immediately after embolization showed thrombi in the proximal portion of both A2 segments. The thrombi appeared to develop by propagation of the thrombi that had formed within the aneurysm (Fig 3B). Bloodflow through the A2 segments was delayed, especially on the right side. The microcatheter was positioned in the mid-A1 segment, and urokinase (150,000 U) was slowly infused. However, this did not produce any change. We decided to use abciximab via an intraarterial route. Initially, 2 mg (1 mL) of abciximab was injected through the microcatheter because the thrombi were small. However, angiograms obtained 5 minutes after injection showed no significant change in the thrombi. An additional 2 mg (1 mL) of abciximab was infused over 1 minute. Ten minutes later, repeat angiography showed more advanced lysis of the clots and full restoration of A2 flow (Fig 3C). Repeat angiography performed 30 minutes later showed the same finding. The patient fully recovered, and no neurologic deficit was observed. Heparinization was continued for 2 days after the procedure. The patient was discharged 14 days later, without neurologic deficit. Aspirin and clopidogrel were administered for 3 months, at which time the patient was free from neurologic symptoms and signs.
Case 3. Angiograms show a thrombotic complication that occurred during embolization with Guglielmi detachable coils; this was successfully treated with intraarterially administered abciximab.
A, Initial angiogram shows an anterior communicating artery aneurysm.
B, Angiogram obtained after complete occlusion with five Guglielmi detachable coils shows luminal narrowings of both A2 segments.
C, Angiogram obtained 15 minutes after intraarterial infusion of abciximab (4 mg) by means of a microcatheter shows an obvious thrombolysis. Bloodflow through the right A2 segment is completely restored.
Discussion
Thrombus consists of multiple elements, including platelets, thrombin, and fibrin. Because thrombolytic agents target only fibrin mesh and because other components of thrombi also play important roles in thrombosis, a complete dissolution of arterial thrombi with thrombolytic agents alone is often difficult. The key factor in the resistance to thrombolytic agents is thrombin (5). If the generation of thrombin exceeds natural anticoagulatory activity, thrombin displays its own activity (ie, cleavage of fibrinogen into fibrin) (6, 7). Undoubtedly, thrombin can be generated without platelets, but only a tiny amount of thrombin is produced by this process. For excessive thrombin generation, the roles of platelets are essential (7). After activation, platelets can facilitate thrombin generation by several mechanisms. First, activated platelets undergo a conformational change that provides a catalytic surface on which thrombin is formed from prothrombin (8). Second, activated platelets release an activated form of factor V from alpha granules (9). Third, activated platelets activate many coagulation factors, such as factors VIII (10), XI (11), and XII (11). Moreover, activated platelets facilitate fibrin mesh formation by activating factor XIII (fibrin stabilization factor) (12) and releasing plasminogen activator inhibitor and vasoconstricting substances (6). In addition, thrombin itself is the most potent stimulant for platelet activation (6). Thus, thrombin facilitates further platelet activation. These processes induce a cascade reaction that causes explosive thrombin generation. Therefore, antiplatelet agents can be used as anticoagulants or thrombolytics. Direct antithrombin agents, such as heparin, may be thought to be the best medication in reversing the effects of excessive thrombin. However, clot-bound thrombin is resistant to antithrombin III (13). Moreover, heparin can paradoxically activate platelets (14).
The most important process of platelet activation is platelet aggregation, and the key element of this process is glycoprotein IIb-IIIa receptor; regardless of the stimulus, the final common pathway for platelet aggregation involves expression of the glycoprotein IIb-IIIa receptors on platelet surface (6). Activated glycoprotein IIb-IIIa receptors have a high binding affinity for fibrinogen and other adhesive proteins that make platelets cross-link one to another (aggregation) (6). All available antiplatelet agents target this process. Aspirin and ticlopidine act by inhibiting arachidonic acid metabolism and adenosine 5′-diphosphate–induced signal transduction, respectively. However, their inhibition of platelet aggregation is incomplete because other pathways can lead to glycoprotein IIb-IIIa activation (15).
Abciximab, which is made by immunization of a mouse with human platelets, is the Fab fragment of a monoclonal antibody directed against the platelet glycoprotein IIb-IIIa receptor. It is the first antiintegrin receptor drug used in humans (15). It is a more potent platelet inhibitor than aspirin (6). The efficacy of abciximab has been extensively documented in experimental as well as clinical studies (1, 7, 16). The most commonly used regimen consists of an intravenous 0.25 mg/kg bolus injection and then a continuous infusion of 0.125 μg/kg/min (maximum 10 μg/min) for 12 hours. This regimen immediately induces an 80% blockade of glycoprotein IIb-IIIa receptors and a prolongation of bleeding time of 30 minutes (6). Inhibition of platelet function is sustained throughout the duration of infusion, and this profound effect persists for 4–6 hours after termination of the intravenous infusion (17).
Recanalization of occluded cerebral arteries is one of the technical challenges in the field of neurointervention. Even after the intraarterial administration of large doses of thrombolytic agent, recanalization is often unsuccessful. A limited time window, potential fatality, and marked benefits from reopening have permitted more aggressive attempts. However, as always, not only the benefits but also the risks should be considered. One of the most serious potential risks is the development of cerebral hemorrhage. In the natural history of cerebral stroke, the risk of hemorrhagic transformation is well known, and the risk can be further increased by adding fibrinolytic agents. In addition, fatality can be increased by fibrinolytic agents (18). In special situations, such as the treatment of thromboembolic complications that develop during coil embolization of ruptured cerebral aneurysms, fibrinolytic agents can be used, but they should be used with caution. Coils cannot replace the volume of the whole aneurysm, and the remaining space is occupied by thrombi, even in completely occluded aneurysms. Because these thrombi are acutely formed, they are easily lysed with fibrinolytic agents. Thus, the aggressive use of fibrinolytics may be dangerous. Moreover, if an aneurysm rebleeds during coil embolization, as in one of our cases, fibrinolytic agents should be used more carefully.
Reducing the dose of a fibrinolytic agent is important. In this situation, the adjuvant use of antiplatelet agents, such as abciximab, can be helpful in the dose reduction. Clinical studies of cardiovascular intervention have shown that the adjuvant use of abciximab permits a reduction in the dose of fibrinolytics and heparin (16). The additional use of abciximab, however, may be dangerous, too. In the treatment of cerebrovascular occlusion, several authors (2, 4) reported cases in which abciximab was systemically used. They showed that abciximab was not only effective but also safe. However, the real safety of abciximab in cerebrovascular interventions has not been established yet. In one study (3) in which abciximab was systematically used, there was a higher frequency of cerebral hemorrhage in the abciximab group than in the placebo group. In a myocardial infarction study (16), a higher dose of abciximab was associated with a higher bleeding rate. Therefore, even though abciximab has been reported as a safe drug, the use of a smaller dose is better. The use of a small amount of abciximab may be more important, especially when the potential fatality of cerebral hemorrhage is high, such as during the acute period of subarachnoid hemorrhage or in association with thrombotic occlusion of major cerebral arteries. In addition, the potential need for emergency surgical intervention may be another reason for reducing the dose of abciximab. From a practical point of view, platelet transfusion is the only method for reversing abciximab. It can rapidly restore platelet action. In general, however, the preparation of platelet concentrates requires time.
We believe that direct intraarterial administration of abciximab is one technique with which the dose of abciximab can be further reduced. Our cases showed successful and rapid recanalization with low-dose urokinase, as well as with low-dose abciximab. We used 4, 5, and 10 mg of abciximab in three cases. Compared with a systemic intravenous dose (the 0.25 mg/kg bolus plus 12-h infusion regimen), they were much smaller amounts (0.14 mg/kg in case 1, 0.05 mg/kg in case 2, and 0.08 mg/kg in case 3). When systemically administered, even a single bolus infusion of 0.25 mg/kg abciximab may be insufficient. This dosage resulted in suboptimal clinical results (1). On the other hand, with intraarterial infusion of even smaller doses, we achieved a prompt reduction of thrombus size or complete disappearance within 5–15 minutes. This means that, even without the saturation of the glycoprotein IIb-IIIa receptors of whole body platelets, locally delivered abciximab facilitates thrombolysis by saturation of glycoprotein IIb-IIIa receptors on the platelets of target thrombi. Theoretically, once glycoprotein IIb-IIIa receptors of locally adhered platelets are saturated, newly replenishing platelets from the systemic circulation cannot initiate the cascade reaction of explosive thrombin generation because platelets must aggregate at the target lesion before their activation, but receptors for the aggregation at the lesion are already blocked. Acute thrombotic complications during vascular interventions develop by means of local intimal injury, not systemic hypercoagulability or systemic platelet activation. Therefore, blocking the glycoprotein IIb-IIIa receptors of locally adhered platelets with intraarterial abciximab infusion may be helpful in other vascular interventions, such as angioplasty or stent placement, that induce platelet activation at a local site. Compared with a systemic dose, a small amount of abciximab is required for receptor saturation at the local site.
Abciximab shows a dose-dependent inhibition of platelet aggregation (6). However, because the state of platelet activation can be influenced by various factors such as the patient’s coagulation status and the local environment of the inner vascular space, the efficacy of abciximab may be different, even with the same weight-adjusted dose. In this situation, dose adjustment may be necessary. In addition, it may be better if the dose can be adjusted according to the time window of the cerebral ischemia. In terms of dose adjustment, the intraarterial use of abciximab has an advantage. For example, when the ischemic time window is sufficient and the thrombus is small, a small amount can be infused first. Because the action of abciximab is prompt, waiting 5–15 minutes is sufficient for checking the effects. If no response is observed, additional abciximab can be infused. When a thrombus is large, relatively large amounts of abciximab can be used first. When necessary, abciximab can be added, up to the weight-adjusted intravenous dose. When the ischemic time window is narrow, high-dose abciximab can be used for rapid thrombolysis, or low-dose abciximab can be used to prevent hemorrhagic complications or to test the effect of abciximab, according to the patient's condition and the physician's decision.
Compared with intravenous administration, intraarterial use of abciximab has several obvious advantages. First, we can deliver high doses of abciximab to the target thrombi; we can therefore expect rapid and effective thrombolysis. Second, we can reduce hemorrhagic complications because small amounts of abciximab and fibrinolytic agents are used. Our patients did not have any bleeding complications, although they all received heparin, urokinase, and abciximab. Third, we can adjust the abciximab dose according to its response. Last, the short action time, although possibly not of merit in cardiovascular intervention, can be attractive in cerebrovascular interventions, especially in rescue thrombolysis.
Conclusion
Three cases of acute thrombosis of cerebral arteries that were resistant to intraarterial urokinase were successfully treated by using an intraarterial infusion of abciximab. Even with small doses, the thrombi were rapidly reduced or completely disappeared within 5–15 minutes. No bleeding complication was observed. We believe that the intraarterial use of abciximab may be promising in effective and safe recanalization of acute thrombotic occlusions of cerebral arteries.
References
- Received June 25, 2001.
- Accepted after revision September 25, 2001.
- Copyright © American Society of Neuroradiology