Abstract
Summary: Infantile dural sinus fistulas are rare and generally have a poor prognosis unless treatment can be undertaken. We report a unique case of an infantile dural sinus fistula with secondary pial recruitment that was managed conservatively. Subsequent spontaneous regression of the lesion occurred over 11 months. The clinical and angiographic features that indicated a probable favorable prognosis are discussed.
Arteriovenous malformations appearing or discovered in infancy are well-described lesions that require the participation of a highly specialized interdisciplinary team for effective and appropriate management. These lesions are dangerous and difficult to treat, and they generally have a poor outcome. Spontaneous regression and resolution of dural sinus fistulas (1, 2) and pial arteriovenous malformations (3) in adults is reported. However, to our knowledge, spontaneous regression of these lesions in infants has not been previously reported.
We present a case of a dural sinus malformation in an infant with angiographic features that may have indicated a poor outcome. The lesion spontaneously resolved over 11 months without clinical sequelae. This case exemplifies that a complete understanding of the lesional angioarchitecture and its effect on the maturing brain is important in predicting the natural history of the disease and in planning appropriate therapy.
Case Report
At 10 weeks of age, a previously well female infant developed a new-onset lateral gaze palsy on the left side. Before the patient’s presentation, the parents had noted that their child had increased drowsiness lasting 48 hours. Clinical examination revealed fixation of the patient’s gaze to the right, with normal pupillary reflexes. A loud left-sided cranial bruit was noted. Physical examination revealed no other abnormality. The patient’s head circumference was in the normal range at the 50th percentile. The intrauterine development and delivery had been unremarkable, and the infant was progressing appropriately along developmental milestones. No evidence of epileptiform activity was noted.
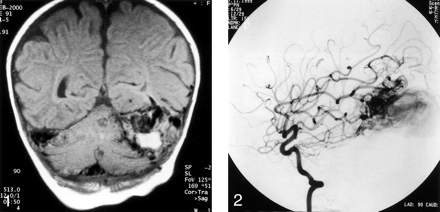
Cranial CT demonstrated an intracranial vascular malformation with a large venous sac. MR imaging results confirmed the presence of a large venous varix containing intraluminal thrombus. This varix was situated between the left temporal lobe and tentorium cerebelli and caused their displacement. Minimal gliosis was present in the adjacent temporal and occipital lobes. No hydrocephalus, cerebral atrophy, or evidence of venous hypertension was observed (Fig 1). Enlarged pial and dural branches supplied the lesion.
Coronal T1-weighted MR image obtained at presentation. A mass is visible between the left tentorium cerebelli and the temporal lobe. Mixed signal intensity is depicted within the mass. The high signal intensity is due to thrombus, and the signal intensity void is compatible with vascular channels. This finding is consistent with a partially thrombosed venous channel. An enlarged right transverse sinus is also shown.
Conventional angiography revealed a complex dural arteriovenous fistula to a large venous sac that was interposed between the tentorium cerebelli and the left temporal lobe. The venous sac freely communicated with a grossly enlarged left transverse sinus. Thrombus filled approximately 30% of the venous sac. No cortical venous reflux or reflux into the superior sagittal sinus was demonstrated. Antegrade cortical venous drainage from the cerebral hemispheres was normal. Mild narrowing of both transverse sinuses was present at their junction with the jugular bulbs, but this did not result in flow restriction. Exocranial outflow occurred via both lateral sinuses and a persistent right occipital sinus.
The major dural supply was via the bilateral middle meningeal, occipital, basal, and marginal tentorial arteries and via the left posterior meningeal artery (Fig 2). The lesion also had substantial pial recruitment from both of the superior cerebellar and posterior inferior cerebellar arteries and from the left middle cerebral artery. Multiple fistulas were present around the venous sac, with rapid arteriovenous shunting.
Lateral left internal carotid angiogram obtained during the arterial phase. The basal and marginal tentorial arteries are enlarged and directly communicate with the venous sac. The secondary recruited pial supply to the dural sinus fistula from the middle cerebral artery is also shown.
In view of the lack of notable clinical deficit, the young age of the patient, and the absence of any high-risk feature associated with the dural fistula (eg, cortical venous reflux), intervention was planned for when the patient was aged 3–5 years, unless clinical or radiologic deterioration occurred. Close clinical and radiologic follow-up was organized.
The gaze palsy resolved within 2 months of its onset. The infant continued to thrive along appropriate developmental milestones.
Follow-up MR imaging and MR angiography performed 11 months after the angiogram was acquired showed almost total resolution of the venous sac, normalization of the size of the left transverse and sigmoid sinuses (which remained patent), and occlusion of the right occipital sinus (Fig 3A). MR angiography showed no arterial supply to the lesion (Fig 3B). No evidence of delayed myelination or atrophy in adjacent brain parenchyma was noted, and no hydrocephalus or acquired Chiari malformation was present to suggest increased venous pressure. Macrocrania did not subsequently develop.
Follow-up MR images obtained after 11 months.
A, Coronal T1-weighted image shows total obliteration of the venous sac, which was previously shown with preservation of the left transverse sinus. The right transverse sinus, which was previously enlarged, has returned to its normal size.
B, Three-dimensional time-of-flight image of the circle of Willis and posterior fossa in the oblique sagittal projection shows no evidence of residual malformation.
Discussion
Dural arteriovenous fistulas in infancy are rare and represent 8% of all pediatric vascular malformations. Conversely, the pediatric population accounts for 11% of all those with dural sinus fistulas (4). Most patients present with high-output cardiac or respiratory failure secondary to cardiac compromise (5–11). Neurologic manifestations are usually the consequences of cerebral venous hypertension and include focal neurologic deficits, hydrocephalus, acquired Chiari malformations (hydrovenous disorders), macrocrania, epilepsy, and hemorrhage (12–14). If these sequelae are not present at the time of diagnosis, the probability of their subsequent development is high. The mortality rate of patients with dural arteriovenous fistulas is reported to be as high as 38% in those with congenital lesions and 67% in neonates (4, 15). Investigators in reported series stress the complex nature of these lesions and the difficulty in achieving an anatomic cure (9, 11, 13–15). Accordingly, the goal of any interventional therapy may be symptom control rather than cure. Inappropriately or incompletely treated lesions may subsequently recruit additional feeding vessels, with resultant angiographic and clinical deterioration that renders further intervention more difficult and hazardous.
Lasjaunias et al (4) previously reported the largest series involving pediatric dural arteriovenous shunts. On the basis of the anatomic and clinical features, they classified congenital dural arteriovenous fistulas into three groups: dural sinus malformation, infantile dural arteriovenous shunts, and adult-type dural arteriovenous shunts. Dural sinus malformations are of two types: type 1 has slow shunting into a giant venous sac that communicates with the major dural sinuses, and type 2 is a direct arteriovenous fistula to the jugular bulb.
The morphologic features of our case most closely correspond with those of a type 1 dural sinus malformation. According to Lasjaunias et al (4), these type 1 lesions are associated with venous hypertension if the venous sac communicates with the venous sinuses across the torcula herophili. The authors state that thrombosis in the venous sac may result in further restriction of the normal cerebral venous drainage. Therefore, occlusion of the venous sac is contraindicated in these lesions. Furthermore, the reduction of venous flow in dilated venous sinuses as a result of shunt closure may lead to spontaneous thrombosis in the sinuses, even if the torcula herophili itself is not compromised. The restricted exocranial outflow that is common in these lesions and due to stenosis or poor maturation of the jugular bulbs may predispose the development of condition. However, in a similar lesion, Komiyama et al (16) reported successful partial embolization of a midline venous sac to stabilize catastrophic cardiac failure.
Maturation of the cerebral venous system is considered critical in predicting a patient’s tolerance to venous occlusion. The cavernous sinus and its connections do not fully develop until 4 months of age. Therefore, infants younger than this have no collateral pathway for diverted cortical venous drainage should the posterior venous drainage be compromised. Therefore, the treatment (palliative or otherwise) of dural sinus malformations in infants of this age should be undertaken with great care because the risk of catastrophic venous sinus occlusion secondary to thrombosis of the dural sinus fistula is increased.
In this case, several features indicated that a favorable prognosis was likely. The lateral position of the venous sac without involvement of the torcula herophili and the absence of direct cortical venous drainage into the sac may have allowed preservation of exocranial venous outflow while the sac underwent thrombosis. Additionally, the lack of direct communication with cortical veins may have been important in preventing the development of venous hypertension as a direct result of either the arteriovenous shunt or the subsequent thrombosis of the venous sac.
There was no angiographic evidence to suggest superior sagittal sinus enlargement or cortical venous reflux. Cross-sectional imaging did not demonstrate any of the typical features of venous hypertension or hydrovenous disorder at presentation or at follow-up. The minor clinical symptoms and signs provides further evidence that venous hypertension was not present at presentation or at follow-up.
The venous sac demonstrated partial thrombosis at angiography. This finding may have indicated that the flow within the sac was relatively sluggish. In addition, the absence of cardiac compromise at any stage suggests that the volume of the shunt was not physiologically substantial.
The anatomic disposition of the venous sac lateral to the torcula herophili is probably the most important factor that resulted in a good outcome. Thrombosis of the venous pouch, by means of either a spontaneous process or embolization, is not expected to result in the development of venous hypertension, because it does not receive antegrade venous drainage from either the cortical veins or the normal venous sinuses. Also, it did not directly communicate with the torcula herophili. The lack of clinical or radiologic evidence of venous hypertension supports the impression that, despite the arteriovenous shunt, the pressure in the venous sac may not have been substantially increased.
The venous sac appeared to become occluded in isolation. The subsequent closure of the occipital sinus is likely to have been physiologic and secondary to the reduction in flow that occurred as the dural sinus fistula became occluded. The exact etiology of the infant’s neurologic deficit remains unclear.
References
- Received November 2, 2001.
- Accepted after revision June 6, 2002.
- Copyright © American Society of Neuroradiology