Abstract
Summary: Although these entities are histologically similar, recent advances in molecular genetics have allowed the distinction of central nervous system extraosseous Ewing sarcoma (CNS-EES) from central primitive neuroectodermal tumors (c-PNET) including medulloblastoma and supratentorial PNET. We present 2 cases of pathologically confirmed CNS-EES. Knowledge of CNS-EES as a distinct entity enables the neuroradiologist to suggest the proper diagnosis and the need for special immuno-histochemical and molecular studies to confirm the diagnosis. Because treatment and prognosis are vastly different, the proper diagnosis of CNS-EES versus c-PNET is critical.
Ewing sarcoma (ES) is usually identified as a primary malignancy of bone with soft-tissue extension. Tefft et al1 described the first series of patients with soft-tissue tumors that histologically resembled ES, with no clear osseous involvement. This group of tumors is currently recognized as extraosseous Ewing sarcoma (EES); it commonly involves the paravertebral regions of the spine and has only rarely been noted to involve primarily the central nervous system (CNS).2 Currently diagnosis of the ES family of tumors is reliably based on MIC-2 antigen expression by immunohistochemistry (IM) and detection of t(11;22)(q24;q12) translocation by fluorescent in situ hybridization (FISH) in tumor biopsy samples.3 This nonrandom translocation is not found in central primitive neuroectodermal tumors (c-PNET) such as medulloblastoma and supratentorial PNET. Although CNS-EES is histologically similar to c-PNET, it differs significantly in clinical behavior, treatment, and prognosis.4,5 In 1999, Jay et al6 described a central nervous system extraosseous Ewing sarcoma (CNS-EES) that was probably the first to be reported in the pathology literature. They described a 4-year-old boy with an isolated posterior fossa mass that demonstrated histologic findings in the spectrum of a c-PNET. However, this tumor was positive for the t(11;22) (q24;q12) translocation by FISH. In addition, we have recently reported, in the pediatric oncology literature, pathologic data on 2 patients with CNS-EES confirmed with IM and FISH analysis.7 So far, 6 cases of CNS-EES confirmed with molecular genetics have been reported in the pathology literature.6,8–11 However, the neuroimaging characteristics of this tumor have not been reported in the radiology literature. We report the radiologic findings of 2 patients with CNS-EES and review the imaging findings of the 6 other cases presented in the pathology literature.6,8–11
Case Reports
Case 1
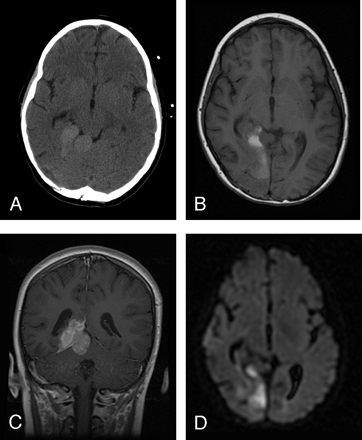
An 8-year-old girl presented with a 4-month history of headache and a 3-week history of nausea and vomiting. No focal neurologic deficits were detected at the time of presentation. A CT scan of the brain without contrast revealed a 2.4 × 3.8-cm hyperattenuated mass centered on the right tentorium cerebelli (Fig 1 A). MR imaging revealed the lesion to be extra-axial in location with a broad tentorial attachment (Fig 1 B). This solitary, solid, homogeneously enhancing mass demonstrated a smooth, lobulated contour, with both infratentorial and supratentorial components (Fig 1 C). Heterogeneous signal intensity on T1- and T2-weighted images was noted with increased signal intensity on diffusion-weighted imaging (DWI) (Fig 1 D). Scattered foci of high T1 and low T2 signal intensity were noted within the central portion of the lesion, thought to be consistent with blood products (Fig 1 B). Findings of further metastatic work-up, including MR imaging of the total spine, CSF cytology, technetium Tc99m bone scan, and thoracoabdominal CT, were negative. Surgical resection via a right occipital craniotomy revealed a purplish, highly vascular tumor. Surgical findings were the broad dural attachment of the tumor along the tentorium and aggressive tumor invasion into the brain parenchyma along all margins. Pathologic evaluation revealed a CNS-EES, which was confirmed by IM analysis to have MIC-2 gene antigen expression and by FISH analysis to have the chromosomal translocation t(11;22)(q24;q12).
A, Unenhanced CT image demonstrates a 2.4 × 3.8-cm hyperattenuated, well-circumscribed, extra-axial mass centered on the right tentorium.
B, Precontrast axial T1-weighted MR image reveals a heterogeneously hyperintense mass with a broad tentorial attachment.
C, Postcontrast coronal T1-weighted image reveals a homogeneously enhancing mass demonstrating a smooth lobular contour, with both infratentorial and supratentorial components.
D, Hyperintense signal intensity on DWI is seen within the medial aspect of the lesion.
Case 2
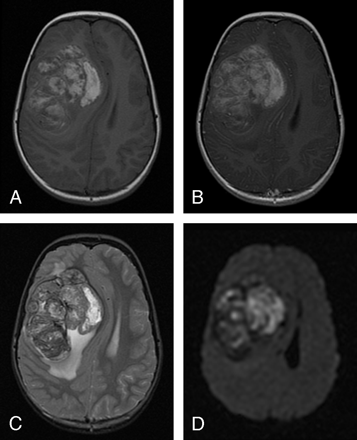
A 7-year-old girl presented with a 1-month history of intermittent headaches, nausea, and vomiting. No focal neurologic deficits were detected at the time of presentation. The brain MR imaging revealed an 8.0 × 8.0-cm medial right frontal lobe mass that appeared intra-axial in location (Fig 2A). However, the medial margin of this lesion was noted to closely approximate the anterior falx cerebri. This lobular mass was predominantly solid with diffuse enhancement and predominantly hyperintense on T1-weighted and heterogeneous on T2-weighted images (Fig 2 A–C). In addition, this lesion was predominantly hyperintense on DWI (Fig 2 D). Several areas of curvilinear central low T2 signal intensity were consistent with blood product deposition. The total spine MR imaging study was negative for tumor metastasis. Further metastatic work-up revealed a 2.8-cm calcified nodule in the left lower lobe on thoracic CT. The pulmonary lesion demonstrated intermediate uptake on a technetium Tc99m bone scan, without any abnormal uptake demonstrated in the skeleton. An abdominal CT scan was negative for tumors. The patient underwent a partial resection of the brain tumor. The tumor was found to be extremely vascular with hemorrhagic components and had an intimate relationship with the dura, necessitating partial removal of the anterior falx to facilitate excision. Pleuroscopy revealed that the lung lesion was intraparenchymal and abutting the visceral pleura. Both lesions underwent IM and FISH analysis and were found to have MIC-2 gene antigen expression and chromosomal translocation t(11;22)(q24;q12), respectively.
A, Precontrast axial T1-weighted image reveals a 8.0 × 8.0-cm predominantly hyperintense lobular medial right frontal lobe mass that was large enough to obscure the distinction between an intra-axial or extra-axial mass lesion.
B, Postcontrast axial T1-weighted image demonstrates diffuse heterogeneous enhancement.
C, Axial T2-weighted image with diffuse heterogeneous signal intensity
D, Predominantly hyperintense signal intensity on axial DWI.
Discussion
ES is an aggressive malignant neoplasm most frequently manifesting in the second decade of life and accounting for 4% of childhood and adolescent malignancies.12 These tumors were first described by James Ewing13 in 1921 as tumors that arise from bone. These osseous lesions have since become infamous for their highly aggressive course with 20% to 30% of patients having evidence of metastasis at the time of diagnosis and an estimated 10-year survival rate of 50%.14 Metastases to the CNS have most recently been estimated to occur in less than 5% of cases and are usually due to direct extension of an osseous lesion into the extradural space or more rarely through hematogenous spread.15 It is predicted that this number will increase with time because of several factors, including increased survival times.15 As recently as the mid 1980s, prophylactic whole-brain radiation was considered an integral component of treatment of osseous ES. However, the risk of such treatment has since been demonstrated to outweigh any marginal improvement in the prevention of intracranial metastasis.16
In 1969, Tefft et al1 described a series of 5 patients with a small round blue cell tumor that histologically resembled ES yet arose from the paravertebral soft tissues. Since then, EES has been recognized as a distinct disease entity that afflicts young adults aged 10 to 30 years, with equal sex predilection.2 This aggressive neoplasm is curable, with the best prognosis observed in patients younger than 16 years of age.2 Histologic examination reveals that these tumors are composed of small, undifferentiated neuroectodermal cells and frequently demonstrate immunohistochemical and/or electron microscopic features of glial or neuronal differentiation.17 In the rare instances in which these lesions arise in the intracranial compartment, they have been commonly misdiagnosed as c-PNET because of similarity in their histologic appearance.9,18 Recent advances in molecular classification of c-PNET and CNS-EES have allowed a clear pathologic distinction between these 2 entities. CNS-EES is now known to demonstrate strong membrane expression of the MIC-2 gene product designated CD99, which is specifically recognized by the monoclonal antibodies O13 and HBA71.19,20 In addition, the chromosomal translocation t(11, 22)(q24;q12) is found in more than 90% of EES. Although CNS-EES is histologically similar to c-PNETs such as medulloblastoma, it differs significantly in clinical behavior, treatment, and prognosis.4,5
Very few cases of proved CNS-EES have been reported in the pathology literature.6,8–11 Jay et al6 were probably the first to describe a patient with an isolated posterior fossa mass that histologically resembled medulloblastoma but demonstrated the t(11;22) (q24;q12) translocation that confirmed CNS-EES. In total, 6 cases of intracranial mass lesions with features of CNS-EES have been reported in the pathology literature, 5 with proved t(11, 22) translocation6,9–11 and the sixth with IM detection of the MIC-2 gene product.5 An additional case of a skull lesion with extension to the intracranial dura in a 2-month-old girl was presented in 1999; however, this case probably represented dural extension from a calvarial Ewing tumor.21
The distinction between CNS-EES and c-PNET is of more than academic interest because the 2 entities require different therapy and carry different prognosis. The treatment options for patients with CNS-EES are similar to those of EES elsewhere in the body and include surgery, chemotherapy, and field radiation therapy. Patients with c-PNET also require surgery; however, the chemotherapeutic and radiation therapy protocols differ from those used for CNS-EES. Because of the small number of patients, the prognosis of CNS-EES is not clearly known, though it has been suggested that patients with EES that arises from structures within or around the CNS may have a more favorable outcome than patients with c-PNET.22 The long-term survival rate for patients with c-PNET is currently only 18%, despite aggressive therapy.5
Our cases share many radiologic features with the 6 previously described cases of pathologically proved intracranial EES (Table). The cases we present reveal a single intracranial lesion. In 1998, Papotti et al11 reported the only known CNS-EES case with multiple intracranial lesions when they described 2 adjacent dural-based frontal region lesions. The second patient we present had a single pulmonary parenchymal metastasis noted at the time of diagnosis. Jay et al6 reported the only other documented case of CNS-EES with known metastasis at the time of diagnosis, and in that instance, spinal drop metastases were seen. Our cases demonstrate lobulated, well-circumscribed margins and diffuse intense enhancement. These features have also been common findings in the cases presented in the pathology literature in which this information was available. The prominent enhancement seen in our 2 patients probably reflects the intense vascularity noted at the time of surgery. A broad dural attachment is another common feature in the cases we present and in all except one of the previously reported cases.6 The second case that we present exhibited a large amount of mass effect, which obscured the relationship of the lesion with the dura. However, this mass demonstrated a broad dural attachment at surgery, which required resection of the anterior falx.
Summary of reported imaging findings
Variability appears to be the rule with both T1 and T2 signal intensity in CNS-EES cases (Table). This may be a result of the frequent occurrence of hemorrhage (2/6 cases in which information was available) and cystic components (2/8 cases). Both cases that we present demonstrate hyperintense signal intensity within the lesion on DWI. The characteristics of diffusion signal intensity within a CNS-EES lesion have not been previously reported, and we anticipate confirmation in future reports. We also note with interest the hyperattenuation on CT of the mass seen in the first patient we present. This appearance has been noted both with hemorrhage and in other tumors with similar attenuated cell packing, as in small round blue cell tumors, such as medulloblastoma. However, the CT attenuation of a CNS-EES has only been presented on one prior occasion,8 and it was noted to be isoattenuated in that instance.
In conclusion, we present 2 cases of pathologically confirmed CNS-EES; recent advances in molecular pathology have allowed the identification of CNS-EES, an entity distinct from c-PNET with which it was previously confused. Several reports of CNS-EES have been presented within the pathology literature; however, to our knowledge, a review of the imaging findings associated with these lesions has not been performed previously. The most consistent appearance is a well-circumscribed, lobular, intensely enhancing, dural-based mass, findings that were present in both of our patients. Variable findings include focal hemorrhage, hyperintensity on DWI, and hyperattenuation on CT. Although this entity is rare, neuroradiologists need to be aware of CNS-EES as a newly recognized distinct pathologic entity with predictable radiographic manifestations.
References
- Received April 1, 2005.
- Accepted after revision May 13, 2005.
- Copyright © American Society of Neuroradiology