Abstract
BACKGROUND AND PURPOSE: Primary lateral sclerosis (PLS) is a rare form of motor neuron disease characterized by upper motor neuron dysfunction. Because pathologic examination has revealed a loss of neurons in the motor cortex of patients with PLS, we sought to confirm and extend this finding by using MR imaging to measure cortical thickness.
METHODS: Seven patients with PLS and 7 age-matched neurologically normal control subjects were examined with heavily T1-weighted short-τ inversion recovery (STIR) MR imaging performed at 3T. Cortical thickness in the anterior and posterior banks of both the central and precentral sulci were measured.
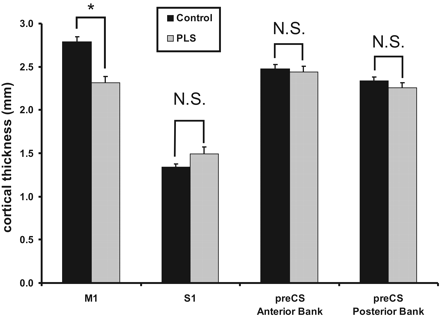
RESULTS: Primary motor cortex (M1) was significantly thinner in patients with PLS than M1 in healthy control subjects, measuring 2.32 ± 0.21 mm compared with 2.79 ± 0.18 mm (P = .0008). Cortical thickness did not differ between the 2 groups for primary sensory cortex or for the anterior or posterior banks of the precentral sulcus. Therefore, loss of gray matter was specific to motor cortex. Although this difference was modest, cortical thickness discriminated between the 2 groups; only 1 PLS case was within the range of normal measurements.
CONCLUSION: Decreased thickness of M1 on the anterior bank of the precentral sulcus in patients with PLS, demonstrable by MR imaging, indicates a selective loss of upper motor neurons in this disease. Measurements of cortical thickness by MR imaging may provide a useful biomarker for diagnosis and study of upper motor neuron diseases.
Primary lateral sclerosis (PLS) is a rare form of motor neuron disease, comprising approximately 5% of all patients with motor neuron disease.1 There has been considerable debate about whether PLS is a variant of amyotrophic lateral sclerosis (ALS) or a distinct disorder.1–3 PLS is distinguished on clinical grounds by a predominant dysfunction of corticospinal neurons of the motor cortex, often termed upper motor neurons (UMN).4–6 ALS is characterized by combined involvement of spinal (lower) motor neurons and UMNs.7
Because the clinical signs of UMN dysfunction, spasticity and hyper-reflexia, can sometimes be masked by dysfunction of lower motor neurons, an objective marker for UMN dysfunction would be clinically useful. Interest has developed in using advanced imaging techniques to improve the detection of UMN abnormalities. Use of diffusion tensor imaging of the corticospinal tract,8–11 voxel-based morphometry,12 and magnetization transfer contrast-enhanced imaging13 have been recently been reported in patients with ALS or PLS.
Pathologic studies of PLS are limited, but some report loss or shrinkage of Betz cells from layer V of the motor cortex.5, 14, 15 Brain MR imaging occasionally shows atrophy in the precentral gyrus in patients with PLS.5 Based on the potential for selective loss of upper motor neurons in patients with PLS, we postulated that the motor cortex would be selectively thinned (relative to neighboring regions of cortex) in patients with PLS, and that this difference would be readily detectable by MR imaging, because the thickness of motor cortex can be reliably evaluated.16 Demonstration of such a reduction in thickness would be important for 2 reasons. First, it would complement the relatively limited pathologic evidence pointing to neuron loss specifically within the motor cortex in PLS. Second, it could provide an objective imaging marker of UMN involvement in PLS. Therefore, we evaluated the thickness of primary motor cortex (M1) by using high-resolution short-τ inversion recovery (STIR) MR imaging at 3T in a group of patients with PLS and compared these measurements to those of matched control subjects.
Methods
Study Population
Seven patients (mean age, 52.9 ± 8.1 years) and 7 age- and sex-matched healthy control subjects (mean age, 53.4 ± 7.4 years) participated in the study (Table 1). All subjects were right-handed. All subjects gave written informed consent for the protocol, which was approved by the Institutional Review Board. The diagnosis of PLS was made using the working criteria proposed by Pringle et al.5 These criteria include clinical signs of corticospinal impairment without lower motor neuron loss, history of insidious progression, laboratory studies to exclude known etiology of progressive spasticity, and lack of family history. All 7 patients with PLS had impaired UMN function in all 4 extremities and bulbar regions, with a duration of disease of 7.9 ± 4.3 years. As an indication of the severity of impairment, finger tapping speed on a keyboard was measured for 3 15-second epochs with the use of a custom software program (LabView, National Instruments, Austin, Tex) and averaged for each hand. Control subjects had normal neurologic examinations and no history of neurologic or psychiatric disease.
Clinical features of patients with PLS
Imaging
Imaging was performed on a 3T MR imaging system. In addition to routine brain imaging sequences, a high resolution STIR sequence was obtained, and a real reconstruction of the data performed to generate heavily T1-weighted contrast. Geometric parameters were 5 mm thick, 1-mm gap, 22-cm FOV, 400 × 320 matrix interpolated to 512 × 512, and an in-plane resolution of 0.43 mm. Contrast parameters were: TR, 4552 ms; TE, 15 ms; TI, 400 ms; echo-train length 5; number of signals averaged, 1, resulting in imaging time of 4:01. Care was taken to align the axial scan plane to be parallel to that defined by the anterior and posterior commissures.
Measurements
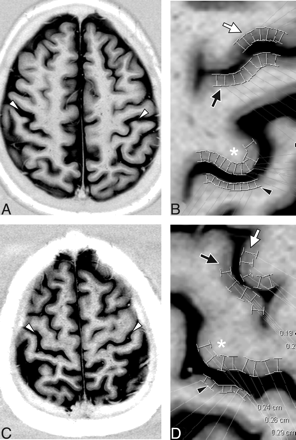
Measurements were made on an axial section at the level of the hand knob using digital calipers on an Ultravisual PACS workstation (version 2.4.2 D; Emageon, Milwaukee, Wis) by a single reader blinded to diagnosis. Cortical thickness was measured to the nearest 0.1 mm using digital calipers on images magnified by a factor of 5 (ie, interpolated to a resolution of 0.08 mm). Measurements were made at 4 sites in each hemisphere for a total of 8 sites per patient. The 4 sites were 1) the primary motor cortex (M1) corresponding to the anterior bank of the central sulcus (CS), 2) the primary sensory cortex (S1) corresponding to the posterior bank of the CS, 3) the anterior bank of the precentral sulcus (pre-CS), and 4) the posterior bank of the pre-CS. At each of these sites, 5–14 (6.6 ± 1.8) individual measurements were obtained. These measurements were spaced ∼2–4 mm apart so that at least a 1-cm extent of cortex was sampled (Fig 1).
Cortical thickness measurements in a 56-year-old woman with primary lateral sclerosis (PLS) (A and B) and age- and sex-matched control subjects (C and D). Real reconstruction of axial 5-mm STIR MR imaging through M1 shows strong gray-white differentiation allowing for identification of the central sulcus (white arrowheads). The neighborhood of the central sulcus for patient (B) and control (D) is magnified from the corresponding images in A and C, respectively. Typical placements of digital calipers in the region of the hand knob (asterisks) for measurement of M1, as well as the measurements of S1 (black arrowheads), and the anterior (white arrows) and posterior (black arrows) banks of the precentral sulcus (pre-CS) are shown. M1 thickness for this patient was 2.16 and for the control subject was 2.63. The ratio of M1 to the pre-CS thickness was 0.92 for this patient and 1.14 for this control subject.
Statistics
Measurements at each site were averaged to generate a single value for each site. Because no asymmetries were expected, values for the 2 hemispheres were averaged in each subject, so that the data were reduced to 4 thickness measurements (1 for each cortical site) in each patient. Cortical thickness measurements were compared with those in the control group by paired t test using Excel (Microsoft, Redmond, Wash). Receiver operating characteristic curves were computed and compared using MedCalc ver. 8.1 (MedCalc Software, Mariakerke, Belgium). All values are reported as mean ± SD.
Results
Measurements of M1, S1, and the anterior and posterior banks of the pre-CS are summarized in Table 2. M1 in patients with PLS was significantly thinner than M1 in control subjects (Fig 2) (2.32 ± 0.21 versus 2.79 ± 0.18 mm; P = .0008). In contrast, no significant difference in cortical thickness between patients with PLS and control subjects was identified in S1 (1.49 ± 0.23 versus 1.34 ± 0.11 mm; P = 0.14), the anterior bank of the pre-CS (2.44 ± 0.19 mm versus 2.48 ± 0.15 mm; P = .68), or the posterior bank of the pre-CS (2.26 ± 0.18 mm versus 2.34 ± 0.12 mm, P = .33). M1 thickness discriminated between patients and control subjects with a high degree of sensitivity and specificity; the area under the ROC curve (AUC) was 0.94 ± 0.071.
Cortical thickness in patients with primary lateral sclerosis (PLS) versus control subjects. There is a significant reduction in cortical thickness in primary motor cortex (M1), but no difference in the other 3 cortices measured. ∗, significant at P = .0008; N.S., not significant; preCS, precentral sulcus; S1, primary sensory cortex.
Absolute and relative cortical thickness measurements in patients with PLS and control subjects
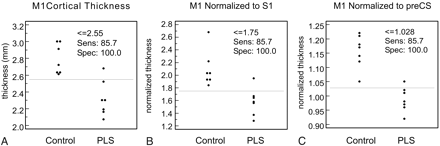
To compensate for individual variation in cortical thickness, we performed 2 different normalizations (Table 2). First, we evaluated the ratio of cortical thickness across the CS; that is, we divided the thickness of M1 by that of S1. Using this normalization scheme, the M1/S1 normalized thickness of patients with PLS was still significantly reduced relative to control subjects (1.58 ± 0.22 versus 2.10 ± 0.28 mm, P = .020) but the level of significance was not as high (i.e., the P value was greater than before the normalization), and there was no change in the AUC of the ROC. The second normalization strategy was to divide the M1 thickness by the averaged thickness of the pre-CS banks. This normalization of M1 cortical thickness to the pre-CS cortices showed a significant difference in normalized M1 thickness (0.99 ± 0.04 versus 1.16 ± 0.06; P = .0005) but at a much higher level of significance (ie, lower P value). A slight improvement in the separation of the 2 groups was seen with normalization (Fig 3), corresponding to an increase in the AUC of the ROC (AUC = 0.99). However, the 2 ROCs were not significantly different (P = .45).
Cortical thickness distribution in control subjects and patients with primary lateral sclerosis (PLS) for M1 cortical thickness (A), M1 cortical thickness normalized to S1 cortical thickness (B), and M1 normalized to the thickness of the anterior and posterior banks of the precentral sulcus (C). Although thresholds with identical sensitivity and specificity can be identified, normalization to the precentral sulcus reduces the overlap between the 2 populations.
No correlation between normalized cortical thickness and duration of disease was found (R2 = 0.1). No correlation between cortical thickness and patient age was found (R2 = 0.05). No correlation between cortical thickness and finger tapping rate was found (R2 = 0.004).
No significant abnormalities were detected on clinical interpretation of the routine brain imaging. In particular, no focal signal intensity abnormalities were identified in the motor cortex or along the corticospinal tracts.
Discussion
Assessment of UMN dysfunction is important in the characterization of motor neuron diseases such as ALS and PLS. Transcranial magnetic stimulation6, 17–20 and MR spectroscopy21–26 are currently the methods most commonly used to assess the motor cortex in patients with PLS or ALS. Each method alone has a moderate sensitivity for detecting UMN abnormalities, with overlapping findings between patients and age-matched healthy control subjects. The combination of both measures has been advocated as a better means to diagnose UMN involvement in motor neuron disease,27 but additional imaging techniques may be another way to improve the detection of UMN abnormalities in ALS and PLS.8–13
Pathologic studies suggest that at least some cases of PLS are characterized by a loss of Betz cells from M1. As regional thinning of the cortex has previously been demonstrated in other neurologic diseases, such as multiple sclerosis28 and Huntington disease, 29 we focused our attention on a direct measurement of M1 cortical thickness. In our group of patients with PLS, all but 1 patient had a cortical thickness lower than the lowest value in the age-matched control subjects. Therefore, patients with PLS are characterized by a selective loss of gray matter in M1.
Primary motor cortex (M1) is readily identifiable on MR imaging by gross anatomic sulcal landmarks in most cases.30 Furthermore, the cytoarchitectonic distinction between M1 on the anterior bank of the CS and S1 on the posterior bank provides a reliable marker that is readily identified on MR imaging.16 Using a STIR sequence at 1.5 T, Meyer et al16 measured the thickness of the cortex on the anterior bank of the CS (M1) to be 2.70 mm and that of the posterior bank of the CS (S1) to be 1.76. In the current study, the thickness of M1, 2.79 mm, was quite comparable, but the thickness of S1, 1.34, was considerably smaller. For the banks of the pre-CS, our measure of 2.41 was also quite similar to that of Meyer et al16 of 2.47.
Several factors could account for the difference in the measurements of cortical thickness in the 2 studies. Higher field strength (3T) alters the T1 relaxation rates of gray and white matter, providing a slightly different contrast in the current study than was obtained at 1.5T. Variations in the plane of section away from the intercommisural plane could introduce random error. The intercommisural plane, though nearly perpendicular to, is slightly oblique to the CS, so that some overestimation of cortical thickness is likely in the absolute measurements for both studies and could result in systematic errors in cortical thickness. In contrast, the ratio of M1:S1 should be relatively resistant to changes in absolute thickness measurements due to section angulation. Perhaps most important is the difference in patient age, 56 years in this study compared with 31 years in that of Meyer et al.16 Finally, because S1 is quite thin and contrast is relatively poor, the error in measurement may be higher. This being said, it is interesting to note that our ratio of M1:S1 of 2.10 is close to the ratio of 2 reported by both Brodmann31 and von Economo and Koskinas.32 However, this variability in measurement is somewhat concerning, because using a specific pulse sequence and field strength limits the generalizability of the technique. It will be important to see whether these results will also be applicable to other high-resolution sequences that provide strong gray-white demarcation, such as magnetization-prepared rapid acquisition of gradient-echo, T1-fluid-attenuated inversion recovery, T1-fast-field echo, and fast echo-spoiled gradient echo.
Many characteristics of the brain, including cortical thickness, vary with extrinsic factors such as age and sex.33 Therefore, some of the variability in the thickness data we measured could be attributable to such external variables. To a first approximation, such variability should in most cases be uniform throughout the brain; therefore, it seemed reasonable to normalize our cortical thickness to other cortical regions that were not expected to be affected by the disease process in PLS. Our initial impulse was to normalize M1 to S1, because the difference in cortical thickness between these regions is quite striking (∼2-fold in our healthy control subjects with the STIR sequence), and the comparison is easily made because the 2 regions span the adjacent sulcus. This normalization had a modest effect on the data distribution. In contrast, normalization of M1 thickness to that of the cortices adjacent to the pre-CS resulted in a greater separation of the control subjects from the patients with PLS, with only 1 case overlapping between the 2 groups. Because of the very small sample, the slight differences in the AUCs were not statistically significant, but the trend toward greater separation of the 2 groups reinforces our belief that some form of normalization of this nature should be performed when examining such brain structures as cortical thickness.
The absolute difference in cortical thickness between the PLS group and the control subjects (0.47 mm) was statistically significant and represented an approximately 20% reduction in cortical thickness across the population. However, this difference is still quite small, approximately the in-plane pixel resolution for the MR imaging data (0.42 mm). Because of this, it may be difficult to reliably demonstrate thinning of the cortical gray matter in any individual patient, even when using normalization. To achieve diagnostic accuracy in an individual patient, obtaining higher resolution MR may be necessary. Another approach is to generate subvoxel accurate surfaces at the gray-white junction and at the pial surface, as can be done from 1 × 1 × 1.5-mm3 voxels (a lower in-plane resolution than used in the current study).34 Such automated measurements allow for cortical thickness measurement accuracies of 0.25 mm and compare favorably to manual measurements,33 and could provide an operator-independent method for objectively measuring the thickness of M1.
Measuring a decrease in cortical thickness provides a direct demonstration of UMN loss in PLS. This has been suggested by prior MR spectroscopy studies, which indicate a decrease in N-acetylaspartate in motor cortex.6 However, the resolution of MR spectroscopy is relatively low, and we believe the data presented here by direct measurement of gray matter thickness to be more compelling evidence. Furthermore, the loss of UMNs in motor cortex can explain the loss of corticospinal tract anisotropy, which has recently been demonstrated by diffusion tensor imaging.9
Since the original description of PLS by Erb35 and Charcot and Joffroy36 more than 130 years ago, 19 autopsies of patients with PLS have been reported, only 6 of these since 1977. In 3 of these 6 cases, a loss of Betz cells from M1 was reported.5, 14, 15 Reduced cortical thickness in PLS demonstrated by MR imaging is consistent with these pathologic studies. Furthermore, we found that reduced cortical thickness was the rule rather than the exception in this group of patients, indicating that loss of neurons in M1 may be an integral feature of PLS, extending the pathologic process beyond the lateral columns of the spinal cord.
Analysis of cortical thickness in M1 and adjacent frontal lobe may provide an ancillary test to confirm the diagnosis of PLS. However, we have only compared this measure with that of healthy age-matched control subjects. It will be important to extend this observation to other diseases that cause cortical atrophy (eg, frontotemporal dementia) and, more importantly, to disease processes that mimic PLS, particularly ALS. In addition, it will be important to extend this morphometric approach to the spinal cord and to examine the relationship between volume loss in the motor cortex and the gray and white matter compartments of the spinal cord.
Conclusions
Selective thinning of motor cortex can be demonstrated in patients with PLS. This is important for 2 reasons. First, it demonstrates that loss of UMNs in motor cortex occurs in PLS, as has been suggested by autopsy reports. Second, it shows that measuring cortical thickness may be a useful biomarker for the assessment of patients with motor neuron disease.
Footnotes
This research was supported by the Intramural Research Program of the Clinical Center of the National Institutes of Health, National Institute of Neurological Disorders and Stroke.
References
- Received October 27, 2005.
- Accepted after revision February 21, 2006.
- Copyright © American Society of Neuroradiology