Abstract
BACKGROUND AND PURPOSE: Periprocedural microembolization is a major and permanent risk for patients treated by angioplasty and stent placement of high-grade carotid stenoses. Little is known however about the characteristics and significance of these embolized particles. Our aim was to assess the volume and composition of debris captured by filters during carotid angioplasty and stent placement (CAS) of severe internal carotid artery (ICA) stenoses.
MATERIALS AND METHODS: Institutional review board approval and informed consent from all subjects were obtained. Two hundred one patients (mean age, 66.2 years; range, 35–82 years) with ≥70% stenosis of the ICA underwent filter-protected CAS. Ultrastructural and semiquantitative analysis of the volume of filters was obtained. Multifactorial statistical analysis was performed to determine factors related to debris volume and composition.
RESULTS: Transient ischemic attack occurred in 6 patients (3%), and a major stroke, in 1 (0.5%). Debris was found in 117 filters (58.2%), with volume <1 λ (0.001 mL) in 71%. The number of balloon dilations, age older than 65 years, and calcified plaques in pre-CAS angiography were significantly associated with the presence of particulates inside the filters (P < .03, P < .004, and P < .05, respectively).
CONCLUSIONS: Vessel wall and atheromatous plaques are the main source of microemboli during CAS. Embolization is mainly related to the number of balloon dilations during CAS. Planning a proper and individualized strategy for the procedure in each patient is essential to minimize the potential effects of manipulation during CAS.
Carotid endarterectomy has been described as the elective treatment for symptomatic atherosclerotic stenosis of the internal carotid artery (ICA), though it is not free of problems such as damage to cranial nerves or complications involving scar tissue.1,2 The development of carotid angioplasty and stent placement (CAS) as a minimally invasive and cost-saving alternative has replaced surgery in centers with extensive experience and skilled interventionists.3,4 However, the chance of ischemic events due to periprocedural microembolization is still a threat to these patients. The advent of cerebral protection devices (CPDs) was greeted with great enthusiasm for reducing the embolic risk. Nevertheless, although CPDs seem safe and effective, silent ischemic lesions secondary to distal embolization have been depicted on diffusion-weighted imaging (DWI) by several authors.5–11
The morphology of the atherosclerotic wall has been the focus of general interest because it has been proved to have a pivotal role in plaque vulnerability.12 The purposes of our study were to describe the method and results of the morphologic analysis of filter content in protected CAS of severe ICA stenosis and to evaluate any correlation found with demographic characteristics, procedural events, and morbidomortality during and up to 30 days after CAS. The results should provide a better understanding of the mechanism of embolization and the potential implication of filters.
Materials and Methods
Study Design
This was a prospective study in a cohort of patients subjected to filtered neuroprotected CAS of ≥70% atheromatous ICA stenosis. Between July 2003 and January 2005, 201 patients were enrolled (166 men, 35 women; mean age, 66.2 years). Symptomatic and asymptomatic patients were included. After each procedure, filters were analyzed macroscopically and ultrastructurally. The study complied with the Declaration of Helsinki and was approved by our institutional review board. Written consent was obtained from all patients.
Study Population
Patients with a ≥70% stenosis according to the North American Symptomatic Carotid Endarterectomy Trial criteria were included.13 Before CAS, neurologic examination and recording of demographic data, symptoms, and vascular risk factors were performed by an independent neurologist (F.M., E.M., J.R.G.-M., or A.G.-P. with 3, 7, 25, and 32 years’ expertise in stroke, respectively).
Intervention Protocol
All procedures were performed by 2 interventional neuroradiologists (A.G., A.M.) with 16 years of experience performing CAS. The details of CAS protocol, together with inclusion and exclusion criteria, can be found elsewhere.14 Briefly, a 6F 90-cm-long sheath catheter was positioned in the common carotid artery (CCA). We used 4 types of filters: EPI and EZ FilterWires (Boston Scientific/Target Therapeutics, Fremont, Calif), Spider (ev3, Plymouth, Minn), and Accunet (Guidant, Santa Clara, Calif). After crossing the stenosis, we deployed a self-expanding basket-type filter with pore sizes 80–130 μm. In all patients, predilation of the stenosis with a 2- to 3-mm balloon catheter was performed, and a self-expandable stent was placed in the ICA-CCA (carotid Wallstent [CWS], Boston Scientific/Target Therapeutics) or carotid Acculink (Guidant). Postdilation was performed with a 5- to 6-mm balloon catheter, when needed.
Patients were on aspirin (125 mg/day) and clopidogrel (75 mg/day) at least for 3 days and unfractionated intravenous heparin for 48 hours before CAS. Electroencephalography (EEG), electrocardiography, and continuous arterial blood pressure were monitored. Hemodynamic changes, such as hypotension (decrease in arterial blood pressure ≥30 mm Hg), bradycardia (≤40 bpm), asystole (cardiac arrest of ≥5 seconds), and syncope (sudden loss of consciousness, usually with additional clinical features and spontaneous recovery), were recorded. Oral clorazepate dipotassium, 20 mg, was used in all patients. Heparin was administered systematically before intra-arterial catheterization to maintain the activated clotting time (ACT) between 250 and 300 seconds. Patients were discharged on clopidogrel (75 mg/day) for a month and aspirin (125 mg/day) or triflusal (600 mg/day) permanently.
Neurologic examination during CAS was performed in all patients and again after 1, 3, 6, and 12 months together with a Doppler sonography of the supra-aortic vessels (F.M., E.M., J.R.G.-M., A.G.-P.).
Morphologic Analysis of Filters
A pathologist (E.R.), with 30 years of experience in neuropathology, reviewed all filters. After extraction, the filter baskets were removed from the guidewire, cut open across their long axes, and examined under stereoscopic microscope. The captured particles were removed under stereoscopic microscope by using microforceps, fixed in paraformaldehyde, embedded in EPON (Resolution Performance Products, Houston, Tex), cut into thin sections, stained with lead citrate, and examined under an electron microscope. Photographs of the device after removal from the guidewire were taken under a stereomicroscope before and after being cut open.
A semiquantitative analysis of the volume of embolic material was performed. A sample was considered to have a maximal tissue load whenever the volume of the captured material was >10 λ (1 λ = 0.001 mL = 1 mm3), a moderate load if the volume was >1 λ, and a minimal score if it was <1 λ (Fig 1).
Volume analysis. Drops are shown with 10-, 5-, and 1-λ volumes. 1 λ is equivalent to 1 μL (0.001 mL) or 1 mm3. The measurement scale is in millimeters.
Statistical Analysis
Data were analyzed with the software package Statistical Package for the Social Sciences, Version 15.0 (SPSS, Chicago, Ill) by a specialist in medical recording with 24 years of experience (A.C.). Qualitative variables were expressed as relative and absolute frequencies, and quantitative variables, as the median and SD. A bivariate analysis was performed with the χ2 test to correlate the volume of debris and its composition, as well as variables such as demographic patient data, vascular risk factors, hemodynamic periprocedural complications, morbidomortality after CAS, with the volume and type of particles. This analysis used the χ2 test for qualitative variables and the Student t test or analysis of variance test for quantitative dependent variables and dichotomies or >2 categories of independent variables, respectively. A P value of < .05 was considered statistically significant.
Results
Two hundred one patients underwent protected CAS of 1 ICA, so a total of 201 arteries were included. One hundred nineteen patients (59.2%) were older than 65 years. Most of our patients were symptomatic (80.1%) with symptoms divided as follows: transient ischemic attack (TIA) in 80 (39.8%), minor stroke in 58 (28.9%), and stroke with minimal residual lesions visible on MR imaging in 23 (11.4%). Hypertension in 74.6% and cigarette smoking in 49.7% were the most frequent vascular risk factors encountered. Symptomatic patients underwent CAS at least 4 weeks after the initial symptoms, but in 13 patients (6.5%), it was performed on an emergency basis. Seventy-nine patients (39.4%) would have been considered high risk for surgery because of heart disease (23.4%), vascular emergency (6.5%), kidney failure (3%), lung failure (2.5%), radiation treatment to the neck (2%), or other risk factors. Angiographic features of the treated arteries are shown in Table 1.
Angiographic characteristics of stenosis
Procedural Events and Clinical Outcome
All procedures were successfully performed in a mean time of 22.2 minutes (range, 8–110 minutes). Filters and stents placed were as follows: EPI in 114 patients (56.7%), EZ in 51 (25.4%), Spider in 27 (13.4%), and Accunet in 9 (4.5%). Regarding stents, CWSs were placed in 139 patients (69.2%), and carotid Acculinks, in 62 (30.8%). In 1 patient, 2 CWSs were used. One or 2 balloon dilations were performed in 146 patients (72.6%). In the remaining 55 patients (27.4%), >2 dilations were needed.
Transient hemodynamic changes occurred in 120 patients (59.7%) without clinical consequences: bradycardia (56.7%), hypotension (49.7%), asystole (35.8%), syncope (27.4%), and EEG anomalies (51.3%). Six patients (3%) developed a TIA lasting <15 minutes. A major hemodynamic contralateral stroke occurred in 1 patient (0.5%) with known occlusion of the contralateral ICA. No other stroke or myocardial infarction occurred in a 30-day period.
Morphologic Analysis of the Filters
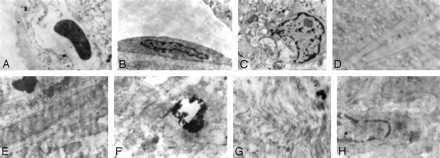
In 117 filters (58.2%), particulates were found (Table 2). In 83 (71%), the volume recovered was <1 λ, and in 27 (23%), >1 λ. In 7 filters (6%), the volume was >10 λ (Fig 2). Fibrin and platelet aggregates outside the filters and attached to the pores were usually found in filters with some content inside the filter (Fig 3). Ultrastructural study of particulate material revealed the presence of fibrin and platelets, foam macrophages, cellular debris, cholesterol crystals, collagen fibers, smooth muscle fibers, calcium, and unidentified fibrillate and amorphous material (Table 3 and Fig 4).
Photograph of a filter with volume content >10 λ before being cut open. The measurement scale is in millimeters.
Photographs of macroscopic views of fibrin and platelet aggregates on the outer surface of filters. A, Plugs of fibrinoplatelet material cover pores outside the filter. B, A cut-open filter shows large-volume particles inside.
Photomicrographs show ultrastructural findings. A, Small capillary. B, Endothelial cell. C, Foam cell with intracytoplasmic inclusions. D, Cholesterol crystals. E, Fragment of the vessel wall. F, Small calcification within the vessel wall. G, Collagen fibers. H, Smooth muscle cell (lead citrate and uranyl acetate, original magnification ×25,000).
Anatomic study of the filter content (201 cases) after CAS
Volume of the particulate material recovered in the distal filter and pathologic composition (117 positive cases)
The number of balloon dilations was significantly associated with the presence of particulates inside the filters (P < 0,03), when 2, 3, or >3 were performed during CAS (Table 4). An association with the particulate volume close to significance (P < .1) was observed. Age older than 65 years and calcified plaques were related to the incidence of captured debris (P < .004 and < .05, respectively). The presence of platelet/fibrin complex, foam cells, cellular debris, amorphous material, and cholesterol clefts was associated with a larger volume of captured material. The type of stent or filter used did not correlate with either the volume obtained or the particulate composition. Sex, vascular risk factors, angiographic characteristics of the stenosis, transient hemodynamic changes, and morbidomortality after CAS were not correlated with the presence of any particulate inside the filter.
Association between the number of balloon dilations performed and filter content
There was a tendency for a higher incidence of TIA or stroke up to 30 days after CAS when debris was found in the filter and when the filters had larger volumes, though without statistical significance (P = .2 and .3, respectively).
Discussion
Distal filters are considered by most authors to be the safest and easiest to use among available CPDs, with results equal to those obtained with balloon-occlusion systems.15–17 Nevertheless, embolization of microparticles through pores with a medium size of 100 μm is a major drawback commonly detected by intraprocedural transcranial Doppler sonography or as new-onset focal ischemic lesions on DWI after CAS.5–11,18–20
Some evidence suggests that mostly the size of particles relates to the occurrence of periprocedural symptomatic neurologic events21–24 and that the brain demonstrates a good tolerance for ischemia caused by these emboli. However, recent reports stress the role that silent ischemia may play in the genesis and maintenance of neurocognitive disorders in these patients.22
Several authors have broadened the investigation of atheromatous plaque and embolization phenomena in the carotid, coronary, and peripheral vascular systems through the direct analysis of particles captured by the filter, trying to corroborate their atherosclerotic origin and establish an association between the volume and composition of the filter particles and postprocedural clinical events and MR imaging findings.21,25–27
In our study, 58.2% of the filters contained debris. Ultrastructural analysis revealed fibrin and platelets, foam macrophages, cellular debris, cholesterol crystals, collagen fibers, smooth muscle fibers, calcium, unidentified fibrillate, and amorphous material. Calcium was the only component related to the capture of larger particles. Our work is one of the scarce reports that inform about the analysis and composition of debris of a very small volume.28 The detected material confirms that vessel wall and atheromatous plaques are the main source of microemboli. These and the predominance of constituents such as cholesterol or fibrin/platelet complexes are the histologic confirmation that supports the concept of patients being prone to embolization (ie, of having vulnerable carotid plaques). This knowledge should encourage us to promote newer techniques of vascular imaging, namely endovascular procedures such as sonography (intravascular sonography) or MR imaging (intravascular MR imaging) to have a better understanding of plaque behavior before interventional procedures, which can simplify and facilitate the selection of patients for CAS.
Most of our filters presented various amounts of fibrin and platelet aggregates in the outer surface as was reported by Sprouse et al.29 They considered this finding to be the cause of pore occlusion. In our opinion, although a main reason for obstruction, it might not be the only cause because we encountered these aggregates on many filters, both with and without particles captured inside. In fact, the cases of Sprouse et al of transient flow impairment due to pore occlusion occurred immediately after postdilation of the stent, when the higher rate of embolization occurs. On the other hand, Castellan et al30 found a significant correlation between the occlusion of the filter and an ACT <380 seconds, reporting a high incidence of fibrin alone inside their filters (38%) and occlusion in almost one third of their patients. In our study, we did not compare these 2 variables because ACT ranged from 250 to 300 seconds in all patients.
A statistically significant relationship was established between the presence and volume of debris and the number of balloon dilations performed. This should be considered a main finding of our study, making us aware of the potential effect of manipulation during CAS and the importance of planning a proper strategy for performing it. The rupture of the plaque after inflation of the balloon before stent application should be a logical explanation for the higher frequency of particle detachment and capture by the filter. A primary stent placement over the stenosis with no previous balloon dilations might be a solution because it would partially sheath the ruptured plaque covered by intima and reduce the amount of debris released, or so it was assumed; Wittkugel et al,28 in contrast, found no difference in debris volume and composition between primary and secondary stent placement in a series of 13 cadaveric specimens.
The volume of debris captured was <1 λ in almost 72% of our patients. Most filters used consisted of a grid with a 100-μm pore diameter mesh. The relatively small percentage of filters in which volumes between 1 and 10 λ were found cannot be exclusively attributed to the passage of particles through the pores because particles with the smallest volume (<1 λ) would have passed much more easily and, paradoxically, were the most frequently captured. Wittkugel et al28 explained a similar finding by fragmentation of particles at the grid of the filter mesh. We should assume that despite the careful handling of filters after CAS, the probability of rupture into smaller fragments is high and can account for the high percentage of small-volume debris encountered.
Authors such as Sprouse et al29 or Mudra et al31 established an association of the capture of debris and several clinical variables (hypertension, hypercholesterolemia), technical factors, or the occurrence of neurologic events after CAS. In our study, a significant relationship between the presence of cell debris and age older than 65 years was the only remarkable finding, which might be explained by the predominance of patients older than 60 years in the study group.
Limitations of the Study
Measurement of the volume and not the bidimensional size of particles can be considered a limitation of our study because it hinders the comparison between series and the understanding of the relationship between the diameter of the pore and the particle main axis.
Capture of particles by filters was significantly more frequent in plaque with calcification detected by angiography because a direct analysis of plaque was not possible. The lack of an in-block excision and histopathologic analysis of the diseased arterial wall makes it impossible to know its real composition and to establish a reliable comparison with the frequency and volume of the debris captured. Further analysis, including direct plaque evaluation in vivo by endovascular imaging techniques, is warranted to arrive at conclusions regarding atherosclerotic plaque behavior and the efficacy of distal CPDs.
In conclusion, we can state that though the use of CPDs during CAS is safe and effective, morphologic analysis of the filters verifies that most captured emboli are constituents of the atherosclerotic plaque and that their release is directly related to manipulation during the procedure. Newer techniques for a better pre-CAS characterization of the vulnerable plaque and thoroughly planned and individualized procedures, with interventionists who have a high level of experience and devices that are easier to handle, should be promoted for improving results.
References
- Received May 14, 2008.
- Accepted after revision October 21, 2008.
- Copyright © American Society of Neuroradiology