Abstract
SUMMARY: This study describes a case of a patient with OSMS who presented with somnolence, periodic fever, memory impairment, and amenorrhea. Serum prolactin levels were found to be higher than normal. MR imaging showed a bilateral involvement of the hypothalamus. The clinical presentation, laboratory examination, and MR imaging findings suggested a diagnosis of hypothalamus syndrome in this patient.
Abbreviations
- AQP4
- aquaporin-4
- CMS
- conventional MS
- CNS
- central nervous system
- EAE
- experimental autoimmune encephalomyelitis
- FLAIR
- fluid-attenuated inversion recovery
- IgG
- immunoglobulin G
- IL-6
- Interleukin-6
- LESCL
- longitudinally extensive spinal cord lesion
- MS
- multiple sclerosis
- NMO
- neuromyelitis optica
- OSMS
- opticospinal MS
The hypothalamus is susceptible to involvement by a variety of processes, including developmental abnormalities, primary tumors of the CNS, vascular tumors, systemic tumors affecting the CNS, and granulomatous diseases. In MS, however, hypothalamus lesions are rare. Herein, we report an unusual case of OSMS with MR imaging abnormalities correlating with hypothalamus dysfunction.
Case Report
A 20-year-old woman first presented 4 years previously with a 5-week history of nausea, vomiting, and hiccups. Physical examination revealed an absent bilateral pharyngeal reflex and a positive Babinski reflex. CSF examination showed normal biochemistry and cell counts. An MR imaging of the brain revealed long T2 signals in the medulla oblongata and posterior horn of the right lateral ventricle. The provisional diagnosis of MS was made despite normal CSF. She responded to a short course of high-dose methylprednisolone, with full neurologic recovery. In the following 4 years, the patient showed a relapsing-remitting course, with clinically estimated main lesions confined to the optic nerve and spinal cord, where the LESCLs, extending over 3 vertebral segments, were found by MR imaging.
Her latest episode started 1.5 months before admission. She presented with hypersomnolence and memory impairment. Her short-term memory was affected, and her attention span was short. She fell asleep very easily in the middle of a conversation and while eating. She missed her period and became ravenous overeating. At the time of neurologic evaluation, the Expanded Disability Status Scale score was 8 with the main involvement of the motor system, sphincterismus disturbances, and memory and arithmetic impairment. An MR imaging of the brain disclosed hyperintense signals in the hypothalamus on FLAIR images (Fig 1). The serum prolactin level was elevated to 52.3 ng/mL (normal range, 3.34–26.72 ng/mL). In the absence of evidence of sepsis, the body temperature remained elevated at 37.5°–38.5°C for 4 weeks. The serum levels of adrenocorticotropic hormone, C3, C4, thyroglobulin antibody, and thyroid microsomal antibody were normal. Tests for autoantibodies, such as C-antineutrophil cytoplasmic antibody, perinuclear-antineutrophil cytoplasmic antibody, anti-SS-A, anti-SS-B, anti-double-strand DNA, anti-Sm antibody, and anticardiolipin antibody, were negative. CSF examination showed elevated IgG (98.8 mg/L; normal range, 0–34.0 mg/L) and immunoglobulin M (3.05 mg/L; normal range, 0–0.3 mg/L). CSF IL-6 level was also elevated (9.47 pg/mL; normal range, 0.01–3.4 pg/mL). The oligoclonal bands in the CSF were not detected. Her serum was tested for the anti-AQP4 antibody by the indirect immunofluorescence method and was negative. Two weeks later, a repeat MR imaging confirmed hypothalamic involvement (Fig 2). The serum ferritin level was elevated to 421.6 ng/mL (normal range, 10.00–150.00 ng/mL). She became less sleepy and better oriented after high-dose methylprednisolone in combination with mitoxantrone; however, her body temperature did not return to normal in the following weeks, and she still had memory and arithmetic impairment and amenorrhea 3 months later.
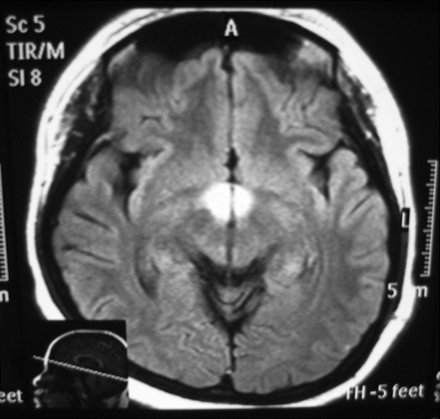
Axial FLAIR image (TR,11000 ms; TE, 120 ms) shows high signal intensity at the hypothalamus.
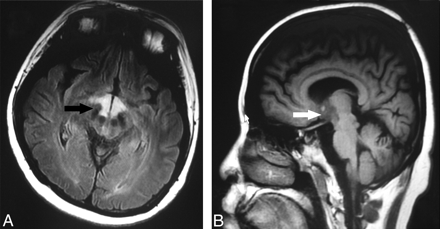
A, Axial FLAIR image (TR, 9002 ms; TE, 160 ms) shows high signal intensity (arrow) at the hypothalamus. B, Sagittal T1-weighted image (TR, 2509 ms, TE, 21 ms) shows low signals (arrow) at the hypothalamus.
Discussion
MS is a demyelinating inflammatory condition of the CNS thought to be caused by autoimmune attacks targeting CNS myelin. In Asians, MS is rare. Reported prevalence rates of MS from population surveys in China are rather low (1–2 per 100,000) and higher in women than in men; these rates are comparable with the results from other populations in Asia.1 In 1996, Kira et al2 first reported that MS in Asians can be divided into 2 subtypes, including OSMS (Asian-type MS) and CMS (Western-type MS) and proposed clinical classification criteria for OSMS: selective involvement of the optic nerves and spinal cord by clinical symptoms with or without minor brain stem signs. Our patient satisfied several characteristic features of OSMS compared with CMS3: absence of oligoclonal bands in the CSF and fewer brain lesions and LESCLs detected by MR imaging.
Optic nerve and spinal cord involvement in our patient made us consider NMO as a differential diagnosis. In 2004, an NMO-specific antibody (NMO-IgG) was found in the sera of patients with NMO, and its target antigen was identified as AQP4 water channel protein.4 The serum anti-AQP4 antibody is thus recognized as a specific biomarker for NMO. Although anti-AQP4 antibodies are present in about 60% of Japanese patients with OSMS over a wide range of disease durations, there are also cases of anti-AQP4 antibody–negative OSMS with LESCLs and few brain lesions.5 Our final diagnosis of this patient was anti-AQP4 antibody–negative OSMS.
Recent data from MR imaging studies suggest that pathologic changes in the deep gray matter, particularly in the caudate nucleus and thalamus, are frequent in patients with MS.6 However, involvement of the hypothalamus is uncommon. In very recent histochemistry, immunohistochemistry, and morphometry research performed on whole coronal sections of 14 MS brains, deep gray matter demyelinating lesions were detected most often in the thalamus (11/14) and caudate (9/14), while hypothalamus involvement was only 4/14, and a preponderance of activated microglia were found in these lesions in the postmortem series of cases.7 Because ferritin can be used as an activation marker of microglia and reflects the extent of inflammation in brain,8 we presumed that microglia activation may exist in the hypothalamus and that elevated serum ferritin might be found in our patient. In agreement with our hypothesis, an increased serum ferritin level was found, which represented active inflammation with ongoing oxidative damage.9 IL-6, which is crucial for the induction of EAE, an animal model for MS, was also found increased in this patient, indicating that she had active CNS inflammation.
Although periodic hypothermia or hyperthermia has been occasionally reported in MS, hyperthemia and disturbances in the sleep-wake cycle and amenorrhea as a result of hypothalamic disease have not been previously reported among subjects with MS. In our patient, systemic causes of hyperthermia were not found. A probable relation of the periodic hyperthermia with a hypothalamic dysfunction is suggested due to the hypothalamic involvement observed in the radiologic study and the abnormal temperature rhythm. Bilateral lesions in the hypothalamus might have provoked the hyperthermia by central rhythm disruption due to excessive heat production or defective heat loss.10 Animal studies have emphasized the importance of the preoptic area of the anterior hypothalamus in the regulation of body temperature and the sleep-wake cycle.11 The patient had no gynecologic disease previously, and her elevated prolactin level and amenorrhea may be explained by impaired hypothalamus-pituitary-adrenal axis activity, which has been described in patients with MS and EAE.12
Acknowledgments
We thank Professor Kai Xu and Dr Xin Lu of the Department of Radiology, Affiliated Hospital of Xuzhou Medical College, who kindly gave us much advice. We also thank Professor Xia Shen and Drs Zhiyong Cao, Cong Wang, Han Liu, Hao Chen, and Fang Peng, Department of Neurology, Affiliated Hospital of Xuzhou Medical College, for their support in data collection.
References
- Received June 24, 2010.
- Accepted after revision July 6, 2010.
- © 2011 by American Journal of Neuroradiology